We engage with you to define panel composition, detection format, donor diversity, and exposure protocol based on the therapeutic's mode of action and ICANS concern.
Immune effector cell-associated neurotoxicity syndrome (ICANS) is a serious adverse event frequently observed with CAR-T and T-cell redirecting therapies. It is driven by aberrant cytokine signaling, endothelial activation, and BBB disruption. Preclinical cytokine release assays, especially those incorporating human PBMCs and multiplex cytokine readouts, provide valuable insights into neurotoxicity risk. Publications support the role of IL-6, IFN-γ, IL-1β, and GM-CSF in ICANS onset, underscoring the need for predictive, human-relevant testing models.
Are you facing challenges in identifying neuroinflammatory responses or predicting cytokine-driven toxicity such as ICANS during immunotherapy development? Our Neurotoxicity Related Cytokine Release Assay Service for ICANS Development helps you assess immune-mediated neurotoxicity risks early through high-resolution cytokine profiling, functional PBMC assays, and single-cell immune response characterization.
Creative Biolabs delivers customizable, validated cytokine release assay services with specific relevance to ICANS and neuroimmune safety:
Services are delivered with full documentation, statistical summaries, and expert interpretation.
By using primary immune cells and high-sensitivity multiplex platforms, our service identifies cytokine release profiles that may signal neurotoxicity risks early in development. This enables developers to modify drug design, dosing strategies, or safety monitoring plans before clinical exposure.
Learn How We Can Support You – Schedule a Consultation
Creative Biolabs offers ICANS-focused cytokine release assay platforms tailored to immunotherapeutic safety evaluation:
We engage with you to define panel composition, detection format, donor diversity, and exposure protocol based on the therapeutic's mode of action and ICANS concern.
PBMCs or fresh human blood are sourced from ethically approved, screened donors. Cell viability, composition, and baseline cytokine levels are assessed before use.
Test molecules are incubated with immune cells at multiple concentrations and time points. Controls (e.g., SEB, anti-CD3) are run in parallel.
Supernatants are analyzed using MSD or ELISA. Intracellular cytokine staining can be included for cell-type specific readouts via flow cytometry.
Clients receive comprehensive reports including cytokine concentration curves, donor variability data, visualizations (heatmaps, PCA), and expert commentary on neuroinflammatory relevance.
Discover the Creative Biolabs Difference – Request Your Quote Today
CAR-T cell therapy initiates a cascade of immune activation involving T cells, monocytes, macrophages, and endothelial cells, all contributing to the pathogenesis of CRS and ICANS. Upon CAR-T engagement, large quantities of cytokines such as IL-6, IL-1, IFN-γ, GM-CSF, and TNF-α are released, promoting systemic inflammation and blood-brain barrier disruption. Monocyte-derived IL-1 and IL-6 are particularly implicated in ICANS development, while endothelial activation and capillary leak further amplify neurotoxicity. Understanding the interplay between immune effector cells and cytokine networks is essential for designing targeted mitigation strategies in CAR-T therapies.
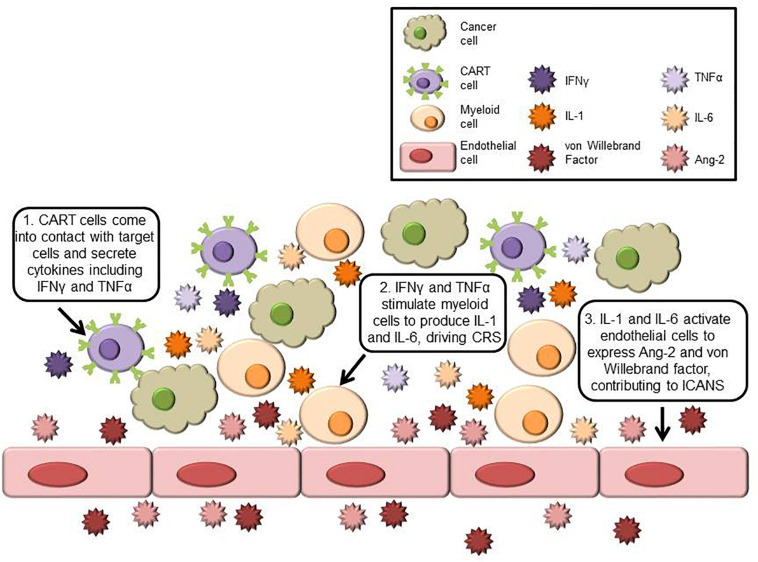 Fig.1 Overview of immune cells and cytokines driving CRS and ICANS.1
Fig.1 Overview of immune cells and cytokines driving CRS and ICANS.1
Can you evaluate both antibody-based and cell-based immunotherapies for ICANS risk?
Yes, our platforms are compatible with monoclonal antibodies, bispecifics, CAR-T, and NK therapies, with adjustments based on mechanism of action.
What cytokines are typically included in your ICANS-related panels?
We commonly include IL-6, IFN-γ, TNF-α, IL-1β, IL-10, and GM-CSF, but panels can be expanded based on your project's immunotoxicity profile.
Do you provide assistance interpreting cytokine signatures?
Absolutely. Our immunologists offer expert data interpretation to help determine neurotoxicity relevance, threshold signals, and next-step recommendations.
How does this compare to in vivo toxicity testing?
In vitro CRA provides early, human-relevant risk signals that complement in vivo models. It is faster, ethical, and often more predictive of CRS and ICANS.
Creative Biolabs provides end-to-end functional CAR-T discovery and development services, including antigen validation, CAR design, cell engineering, and in vitro/in vivo efficacy testing to accelerate the creation of potent, tumor-targeted cell therapies.
Our cancer vaccine formulation development service offers customized adjuvant selection, delivery system optimization, and stability assessment to create effective and scalable immunotherapeutic vaccines tailored to diverse tumor types.
Creative Biolabs empowers immunotherapy developers with precise, customizable, and human-relevant cytokine release assay platforms to predict and prevent ICANS.
Contact Our Team for More Information and to Discuss Your Project
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION