The clinical application of NK cells is often constrained by the limitations of conventional expansion methods, which frequently yield products with insufficient cell numbers, functional exhaustion, and impaired in vivo persistence. To address these challenges, Creative Biolabs' Nicotinamide-Induced NK Cell Expansion Technology provides a novel platform that fundamentally enhances NK cell fitness through metabolic reprogramming. By modulating cellular metabolism, Nicotinamide (NAM) not only promotes robust, large-scale ex vivo expansion but also concurrently upregulates key activating receptors and augments mitochondrial fitness, resulting in NK cells with superior cytotoxicity and enhanced survival in the immunosuppressive tumor microenvironment.
NK cells are potent effector lymphocytes vital for cancer immunosurveillance. Their innate tumor-killing capability and low risk of make allogeneic cell therapy highly promising. However, clinical efficacy has historically been limited by poor expansion yields and short persistence due to metabolic exhaustion. NAM, a form of vitamin, solves this by acting as a kinase inhibitor and metabolic conditioner. By stabilizing the transcription factor, enhances the expression of selectin and glucose flux, resulting in a robust, high-purity product with superior persistence and resistance to tumor evasion mechanisms.
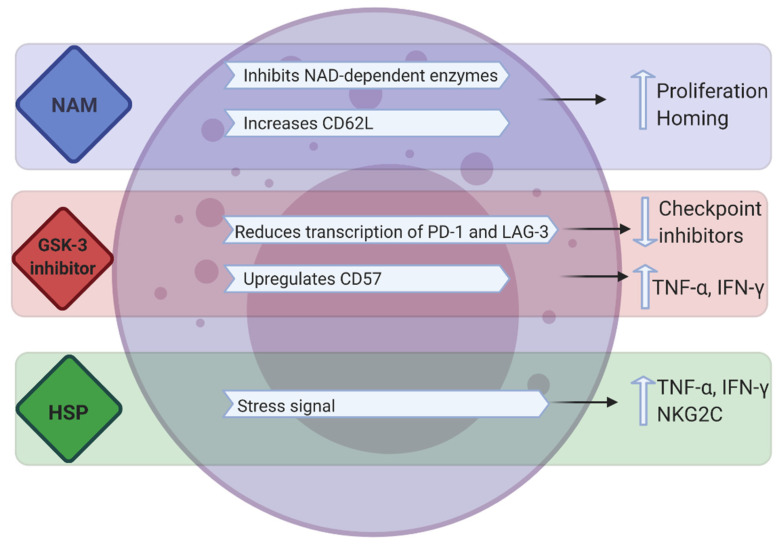 Fig.1 Modulation of NK cell function through NAM, GSK-3 inhibition and HSPs.1
Fig.1 Modulation of NK cell function through NAM, GSK-3 inhibition and HSPs.1
Creative Biolabs' Nicotinamide-Induced NK Cell Expansion Technology provides specific, high-quality deliverables by leveraging metabolic and kinetic controls to produce reliable cells. We deliver a product that is not only highly expanded but is functionally pre-conditioned for superior activity within the immunosuppressive tumor microenvironment.
We offer a complete customizable platform designed to deliver reliable and effective cell products and development services tailored to your specific oncology targets.
We implement a streamlined, multi-stage process designed for maximum yield, high purity, and optimal functionality, making the resulting cell products ideally suited for downstream engineering or direct clinical application.
Required starting materials:
Key Steps Involved:
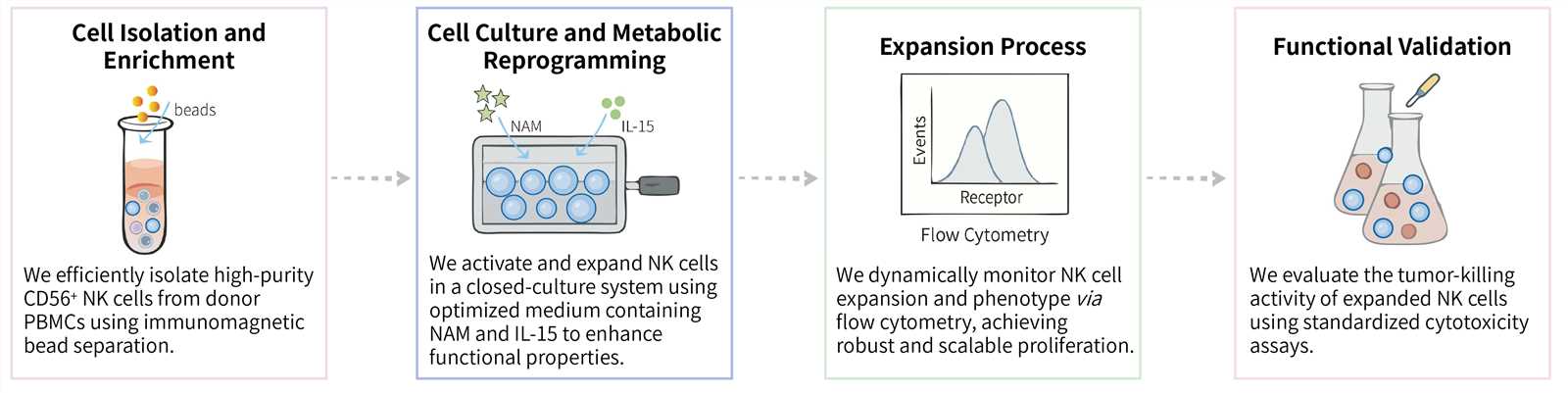
Final Deliverables:
What is the benefit of NAM acting as a kinase inhibitor during the expansion process?
NAM inhibits kinases like ROCK and CK1, suppressing actomyosin contraction. This is crucial during cell processing steps, such as mRNA electroporation for CAR introduction, as it dramatically improves cell survival and viability post-manipulation, translating directly into higher manufacturing yields and greater final product quality.
Can your NAM-expanded NK cells be genetically modified?
Of course. Our NAM platform provides an ideal, highly viable, and large-scale starting material for advanced genetic modification. We routinely integrate CAR expression and use technology to engineer enhancements, such as deleting inhibitory markers like CD38 on the NK cells themselves to prevent fratricide. Inquire today to discuss integrating custom genetic edits into your NAM-NK product.
Moving beyond simple expansion, our platform is dedicated to engineering the next generation of therapeutic cells through advanced metabolic and genetic reprogramming. We specialize in the creation of metabolically and genetically optimized cellular products, engineered for enhanced potency, persistence, and clinical efficacy. Our integrated platform is built upon a foundation of evidence-based mechanisms and rigorously validated protocols, ensuring a streamlined and de-risked pathway from preclinical discovery to clinical-scale manufacturing.
"The low-cell contamination we consistently observe with Creative Biolabs' Nicotinamide-Induced NK Cell Expansion Technology, contrasted against previous feeder-cell or protocols, is a major advantage for readiness. It eliminates significant downstream purification bottlenecks."— L. B*****tt.
"Using Creative Biolabs' Nicotinamide-Induced NK Cell Expansion Technology in our research has significantly improved the expression profile, which is critical for our xenograft models where superior lymph node homing is required. The consistency is unmatched."— J. S*****an.
To address the critical translational challenges of cell yield and durability for your specific program, connect with our specialists to define a tailored development path.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

