γδ T cells represent a potentially transformative advance in the treatment of malignant diseases. The development of gene-modified γδ T cells is a promising strategy in cancer immunotherapy. Aiming to fully support customers and advance the development of cell immunotherapy, Creative Biolabs provides comprehensive services to analyze and assess the function of NKT TCR-γδ T cells.
The approach of NKT TCR transfection into γδ T cells has a number of unique features that make it attractive for use as an immunotherapeutic approach to cancer treatment. The use of TCR-transfected γδ T cells could be a promising alternative for cancer immunotherapy.
NKT TCR engineered γδ T cells can recognize both the NKT cell and the γδ T cell ligands. After stimulation with ligands for αβ and γδ TCRs, NKT TCR-γδ T cells rapidly proliferate, produce cytokines and generate cytotoxicity against tumor cells. NKT TCR-γδ T cells show enhanced killing activity against the target cells. However, these cells have limited persistence in vivo, that be beneficial in attenuating cytokine release syndrome and neurotoxicity.
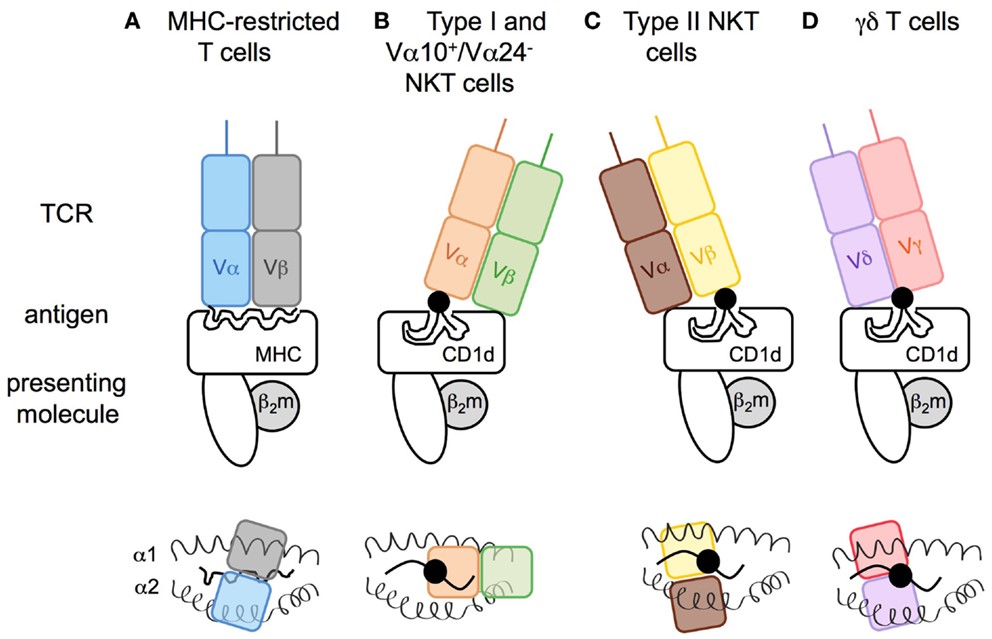 Fig.1 Modes of antigen recognition for CD1d-restricted T cell receptors. (Macho-Fernandez, 2015)
Fig.1 Modes of antigen recognition for CD1d-restricted T cell receptors. (Macho-Fernandez, 2015)
To assess whether NKT TCR-γδ T cells respond to both the NKT/ γδ ligand, these cells are tested the proliferation and IFN-γ production after the phosphoantigen or α-GalCer stimulation as compared to control parental γδ T cells. Cytotoxicity assay is used to evaluate the antitumor effect by testing NKT TCR- γδ T cells against target cells that express phosphoantigen or α-GalCer.
At Creative Biolabs, our scientists and technicians can provide comprehensive assays to assess the NKT TCR-γδ T cell-specific response to both the NKT/ γδ ligand. Our experienced scientists and technicians can provide comprehensive assays including cytokine release assay, cytotoxic assay and cell proliferation assay. You can pick your targets of choice from our catalogue to set up your personalized panel.
Benefits of our services include:
If you are interested in our services, please feel free to contact us and discuss your customized needs with our scientists.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION