Chimeric antigen receptor (CAR) T cell therapy has been at the forefront of revolutionary cancer treatments, particularly within the field of hematological malignancies. Traditional CART preparation methods in vitro involve a multi-step, arduous procedure, starting with leukapheresis to harvest a patient's T cells, followed by artificial activation, gene incorporation, and cell expansion. This preparation process requires the application of a variety of reagents, thus emphasizing the need for a more streamlined and efficient approach. At Creative Biolabs, we have developed a powerful one-step activating lipid nanoparticles (aLNPs) technology for mRNA CAR T cell development, promising to simplify this complex process.
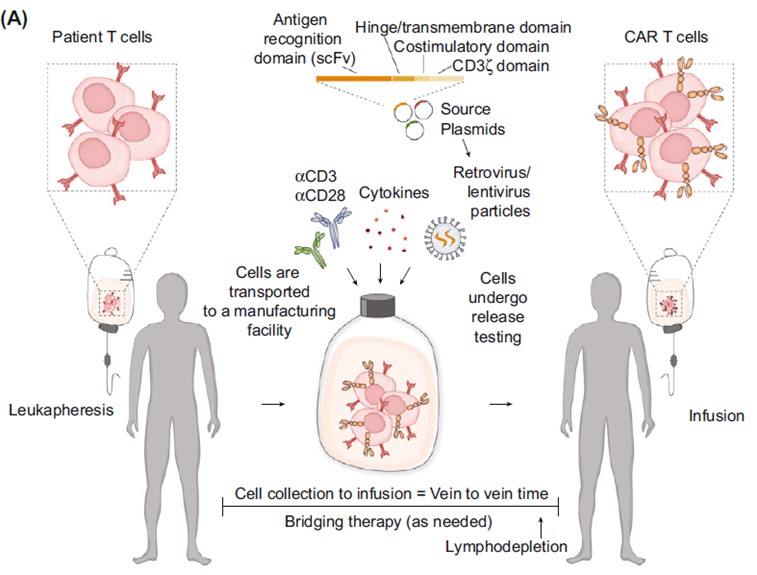 Fig.1 Ex vivo manufacture procedure of CAR T cell product.1
Fig.1 Ex vivo manufacture procedure of CAR T cell product.1
Our innovative aLNP technology leverages the synergistic combination of nanoparticle simulation of mimic antigen-presenting cell (APC) activation and LNP transfection performance. Traditional methods use antibody-conjugated magnetic beads targeting CD3 and CD28 receptors to activate T cells, which need to be removed after activation, thereby extending production time and increasing complexity. We have addressed this bottleneck by designing lipid nanoparticles that bind to CD3 and CD28 antibody fragments, effectively eliminating the need for activation beads and enabling a streamlined one-step process.
The aLNPs are meticulously engineered using a thiol-maleimide reaction to attach CD3 and CD28 antibody fragments onto the nanoparticle surface. These nanoparticles can simultaneously activate and transfect T cells with CAR-encoding mRNA, thereby bypassing the traditional multi-step activation process. This design ensures localized delivery and expression of CAR constructs, priming the T cells for immediate functionality upon reinfusion.
The aLNP technology transforms the CAR T cell manufacturing process by consolidating activation and transfection into a single step. By obviating the need for magnetic beads, the workflow is reduced from 48 to 24 hours, significantly cutting down both the production time and operational complexity.
Utilizing multiple screening libraries we already have, such as advanced phage display libraries, to generate high-affinity scFv that can accurately identify peptide/HLA complexes. We will further optimize these scFvs to ensure stability and binding efficacy.
With fewer steps and lesser need for specialized equipment like magnets for bead removal and electroporation devices, the production costs are considerably lowered.
The transient nature of mRNA avoids the risks associated with genome integration that are inherent to viral vectors. This non-viral delivery system also minimizes cytotoxicity and off-target effects, ensuring a safer profile for clinical applications.
Q1: What is the primary advantage of using aLNPs over traditional CAR T cell development methods?
A1: The primary advantage lies in the consolidation of activation and transfection steps into a single streamlined process. This reduces procedural time, complexity, and costs, making CAR T cell therapy more accessible and scalable.
Q2: How does the aLNP technology impact the safety profile of CAR T cell therapies?
A2: Because aLNPs utilize mRNA instead of viral vectors, there is no risk of genome integration, which significantly reduces long-term side effects and cytotoxicity. This makes the therapy safer for patients.
Q3: Can aLNPs be used for purposes other than cancer treatment?
A3: Yes, the versatility of aLNPs allows them to be potential candidates for treating autoimmune diseases, delivering different types of therapeutic mRNAs, such as those encoding cytokines or growth factors, and even for combination therapies involving immune checkpoint blockade.
Creative Biolabs' One-step Activating Lipid Nanoparticles technology represents a significant advancement in the field of mRNA CAR T cell development. This innovative approach not only streamlines the production process but also reduces costs and enhances the safety profile of CAR T cell therapies. If you would like to know more about our technology, please feel free to contact us for direct communication.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION