TILs represent a promising avenue in the realm of cancer immunotherapy, leveraging the body's immune cells to target and eradicate malignant cells. As the field of TIL therapy continues to evolve, understanding the pharmacokinetics and toxicology of TIL products is paramount for ensuring their safety and efficacy.
Creative Biolabs specializes in comprehensive pharmacokinetics (PK) and toxicology studies tailored for TIL therapy development. Our approach integrates advanced methodologies to assess the behavior of TILs within the body, including their distribution, metabolism, and excretion. By utilizing state-of-the-art techniques, we can provide detailed insights into how TILs interact with various biological systems, which is crucial for optimizing treatment protocols and enhancing therapeutic outcomes.
One of the key components of our service is the evaluation of the pharmacokinetic profiles of TIL products. To measure the pharmacokinetic characteristics of input cells, several in vivoexperimental methods can be employed:
| Nuclear Imaging | This method allows for the rapid detection and quantification of target cells, although it cannot distinguish between live and dead cells. |
| Magnetic Resonance Imaging | This technique involves collecting limited tissue samples for analysis and requires specific antibody labeling, which may affect accuracy and stability. |
| Optical Imaging (BLI; FLI) | This method allows for real-time tracking of target cells by detecting emitted light from labeled cells. |
| Photoacoustic Imaging | An emerging non-invasive imaging modality that combines optical and ultrasound imaging to provide high-resolution images of target cells in real time. |
| Ultrasound Imaging | This technique can be used to monitor the distribution of target cells in tissues, providing a non-invasive method for tracking. |
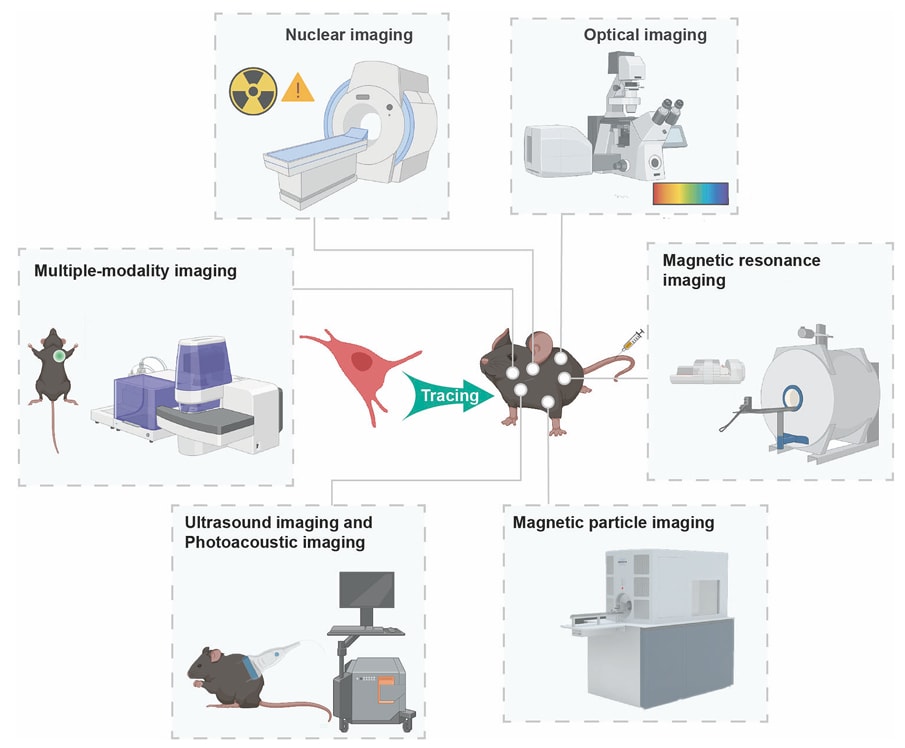 Fig. 1 Various imaging and tracking technologies in monitoring the migration and distribution of MSCs.1
Fig. 1 Various imaging and tracking technologies in monitoring the migration and distribution of MSCs.1
Flow cytometry also plays a key role in PK studies primarily by measuring cellular kinetics, particularly in monitoring the dynamic changes of infused cells after adoptive cell therapies. Unlike traditional pharmacokinetics for small and large molecules, flow cytometry can assess the proliferation capability of these "living drugs", as they can proliferate in vivo after infusion. PK studies allow researchers to fine-tune dosing regimens and improve the overall effectiveness of TIL therapies.
Creative Biolabs' toxicology studies are designed to identify any potential safety concerns associated with TIL therapies. We conduct extensive assessments to evaluate the immunogenicity and potential off-target effects of TILs, ensuring that any adverse reactions are thoroughly documented and understood. This includes monitoring for cytokine release syndrome and other immune-related adverse events, which are critical considerations in the context of TIL therapy.
Creative Biolabs' commitment to advancing TIL therapy is reflected in our collaboration with clinical researchers and pharmaceutical companies. By providing robust pharmacokinetic and toxicology data, we aim to facilitate the transition of TIL products from preclinical stages to clinical trials, ultimately contributing to the development of safe and effective cancer treatments. The pharmacokinetics and toxicology study service at Creative Biolabs for TIL therapy development is dedicated to providing comprehensive, high-quality research that supports the safe and effective use of TILs in cancer treatment. As we continue to explore the potential of TILs, we remain committed to advancing the science of immunotherapy. Reach out to us for more information on pharmacokinetics and toxicology for TIL therapy development.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION