All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Viral vector plasmids play a crucial role in the development and production of CAR-T cell therapies, which have emerged as a groundbreaking approach in cancer treatment. These plasmids serve as vehicles for delivering chimeric antigen receptor (CAR) genes into T cells, enabling the modification of these immune cells to target and eliminate cancer cells effectively.
The primary types of viral vectors used in CAR-T cell production are lentiviral vectors and retroviral vectors. Lentiviral vectors are particularly favored due to their ability to integrate genes into the genomes of both dividing and non-dividing cells, making them versatile for various cell types, including T cells. They offer several advantages, such as efficient gene integration, stable expression of target genes, and a lower risk of oncogenesis compared to retroviral vectors. This makes lentiviral vectors extensively used in clinical trials and CAR-T cell production.
On the other hand, retroviral vectors primarily transduce dividing cells, which limits their application in certain scenarios. While they are effective in specific contexts, their higher risk of oncogenesis compared to lentiviral vectors restricts their widespread use. Nonetheless, both types of viral vectors are integral to the CAR-T cell production process, ensuring that the modified T cells can effectively express the CAR and target cancer cells.
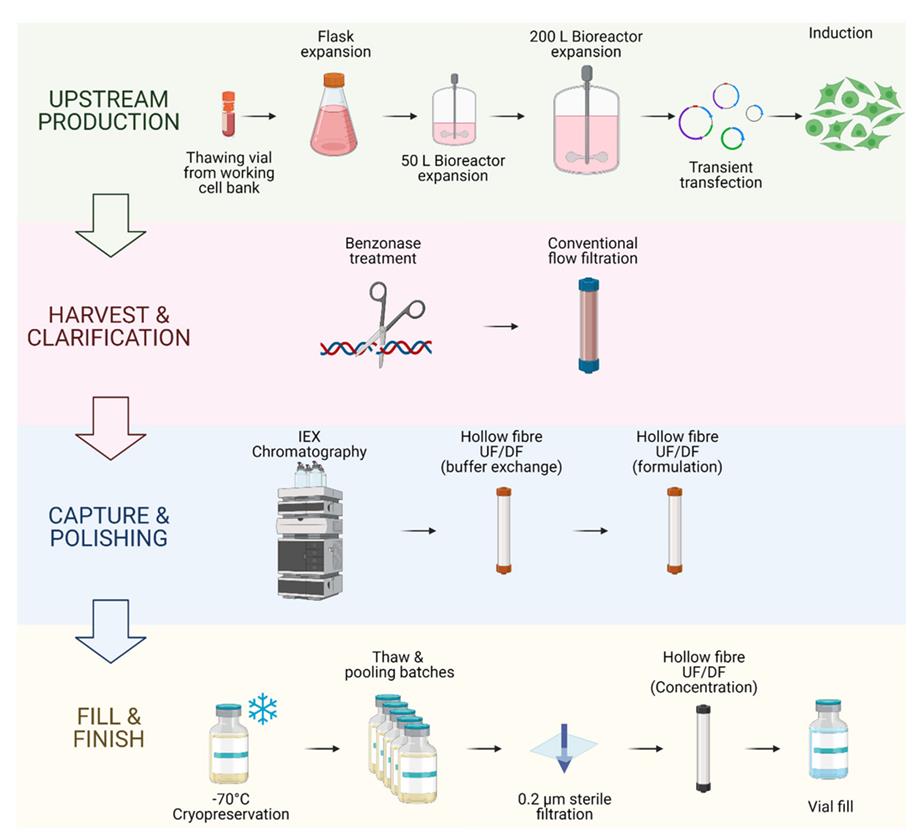 Fig. 1 A comprehensive view of the entire GMP-grade lentiviral vector production process.1
Fig. 1 A comprehensive view of the entire GMP-grade lentiviral vector production process.1
The production of viral vector plasmids must adhere to stringent quality standards to ensure safety and efficacy. Good Manufacturing Practice (GMP) guidelines are essential in this regard. GMP vectors are produced under controlled conditions that minimize contamination risks and ensure product consistency. This is critical for clinical applications, as the quality of CAR-T cells directly impacts their therapeutic efficacy and patient safety. GMP-compliant processes involve meticulous production, purification, testing, and quality control measures, which are vital for the successful generation of CAR-T cells.
Creative Biolabs offers a range of viral vector plasmids, including GMP-like and GMP-grade products. GMP-like vectors serve as an intermediary between research-grade and full GMP-grade vectors, tailored for preclinical studies. These vectors are produced under an ISO9001 quality system, ensuring a level of quality control suitable for preclinical applications. They provide a more economical and time-saving option compared to full-scale GMP production, making them ideal for drug discovery and testing.
In addition to GMP-like products, Creative Biolabs also provides off-the-shelf plasmid products. These pre-prepared plasmids are designed for large-scale production, significantly reducing production time and improving cost-effectiveness. The standardized production process ensures consistency in plasmid quality, enhancing the reliability and safety of CAR-T cell products. Off-the-shelf plasmids are ready for immediate use, facilitating quick access for researchers and developers, and are suitable for various stages of research and development, from preclinical studies to clinical trials.
We welcome all researchers and developers interested in CAR-T cell therapy and viral vector plasmids to contact us. Creative Biolabs is dedicated to providing high-quality customized solutions to meet your specific needs in gene and cell therapy development. Whether you are looking for GMP-like products, GMP-grade plasmids, or off-the-shelf plasmid products, our team is here to offer support and professional advice. Please feel free to reach out to us to discuss your project requirements. We look forward to collaborating with you to drive innovation and advancement in cancer treatment.
Reference
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION