Creative Biolabs' excellence in Contract Development and Manufacturing Organization (CDMO) services is underscored by our sophisticated and innovative approach to process development, particularly in the emerging field of CAR-T cell therapies. As the landscape of cell therapy evolves, the demand for robust, scalable, and regulatory-compliant processes has never been greater. Our commitment to leveraging cutting-edge technology and our extensive experience ensures the delivery of high-quality CAR-T cell therapies to the market.
At Creative Biolabs, process development for CAR-T cell therapies begins with a meticulous understanding of each compound's unique requirements to design, refine, and optimize processes that drive efficiency and quality. Our technology-agnostic capabilities and state-of-the-art facilities guarantee the development of processes tailored to transition seamlessly from the research scale to large-scale GMP manufacturing.
Early to Late-Phase Development
We adopt a phase-appropriate approach that evolves according to the needs of each development stage. In the early stages, we focus on accelerating the journey to clinical trials using science and innovation. Safety, quality, and sustainability are paramount as we ensure faster turnaround times without compromising on these aspects. For late-phase development, our expertise in developing several molecules for commercial markets like the USA, Europe, and Japan equips us with a robust and systematic process development approach. By using a Quality by Design (QbD) methodology, we ensure that key process parameters impacting product specifications are thoroughly understood and controlled.
Creative Biolabs offers end-to-end solutions for process development:
Our upstream process development is facilitated by advanced biomanufacturing facilities equipped with scalable fermenters and single-use bioreactors. Activities like cell culture, media screening, process optimization, product quality modulation, and process scale-up are conducted with utmost flexibility and precision.
We focus on enhancing product purity through sophisticated purification and separation methods. The aim is to eliminate product-related, process-related, and adventitious impurities. Our tailor-made downstream processes meet stringent quality standards set by customers and regulatory bodies.
Formulation development at Creative Biolabs ensures the stability and preservation of biological products for long-term storage and optimal therapeutic effectiveness. By standardizing our formulation development service process, we effectively reduce drug development time while ensuring patient safety.
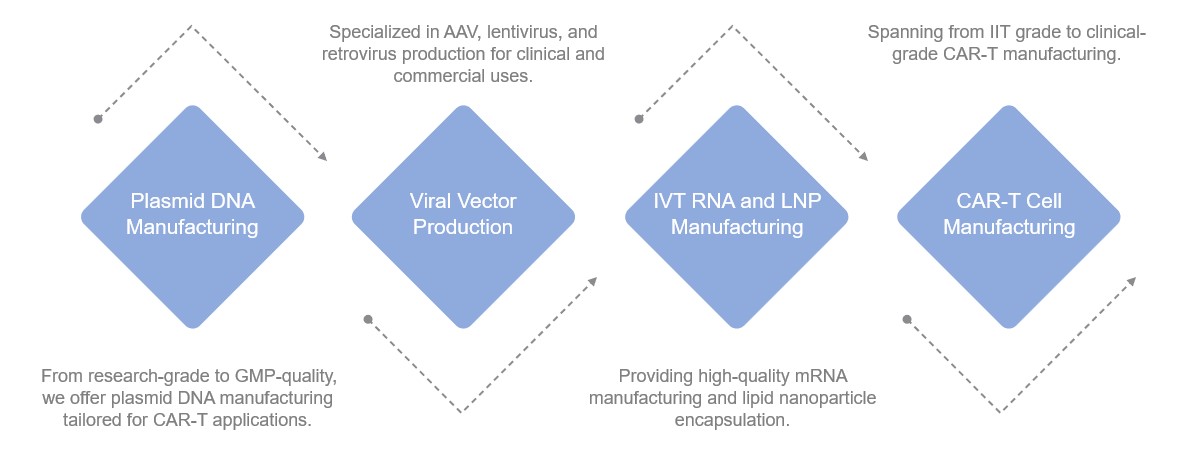
Creative Biolabs provides comprehensive CAR-T related services as follows:
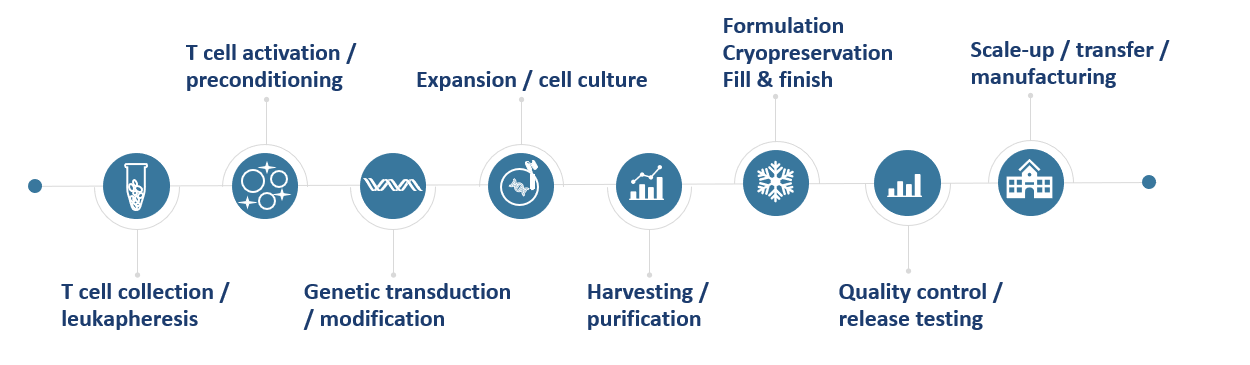
Below presents Creative Biolabs' typical workflow for global CAR-T CDMO process development, including core stages like process design, testing & refinement to boost therapy development efficiency.
Across its various technology platforms, Creative Biolabs has established suitable quality control methods, with quality control tests including, but not restricted to, the following content.
| Category | Assay | Analytical Techniques |
|---|---|---|
| Identity and Purity | Cell Phenotyping | Flow Cytometry |
| Genetic Verification | PCR / Quantitative Polymerase Chain Reaction | |
| Vector Integrity | DNA Sequencing (Sanger Sequencing, NGS) | |
| Residual Cell Contaminants | Sanger Sequencing, NGS | |
| Functionality | In Vitro Cytotoxicity | LDH release assay, T Cell-mediated Tumor Cell Lysis Assay |
| Cytokine Secretion | ELISA / Multiplex Cytokine Panels | |
| CAR Expression | Flow Cytometry | |
| Proliferation & Persistence | Flow Cytometry / CFSE labeling | |
| Safety and Biosafety | Sterility | Microbial Culture, Gram Staining |
| Mycoplasma Detection | PCR / culture-based Assays | |
| Viral Safety | Replication Competent Lentivirus/Retrovirus Testing | |
| Residual Process Contaminants | Sandwich ELISA | |
| Endotoxin and Pyrogens | Limulus Amebocyte Lysate Assay |
Get in touch with our team for additional details and project consultation.
How does Creative Biolabs ensure the scalability of CAR-T manufacturing processes?
Our phase-appropriate approach and advanced biomanufacturing facilities enable us to design scalable processes. By leveraging our experience in both fed-batch and perfusion production modes, we can meet varying productivity requirements and maintain product quality.
What regulatory standards do you adhere to in your process development?
Creative Biolabs adheres to global regulatory requirements and ICH guidelines. Our commitment to safety and compliance ensures that our processes meet the stringent standards set by regulatory bodies.
Creative Biolabs remains at the forefront of process development for CAR-T therapies, offering comprehensive, innovative, and regulatory-compliant solutions that drive the successful commercialization of advanced therapeutics.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION