The effectiveness of CAR-T therapy heavily relies on the quality and consistency of the viral vectors used for T-cell modification. This necessitates high-quality, Research Grade (RG) virus manufacturing processes to ensure the safety, efficacy, and reproducibility of these therapeutic products. At Creative Biolabs, we focus on delivering cutting-edge virus manufacturing services specifically designed for CAR-T therapy. Our state-of-the-art facilities and experienced team leverage cutting-edge technologies to produce high-quality viral vectors, ensuring optimal transduction efficiency and safety for CAR-T cell therapies.
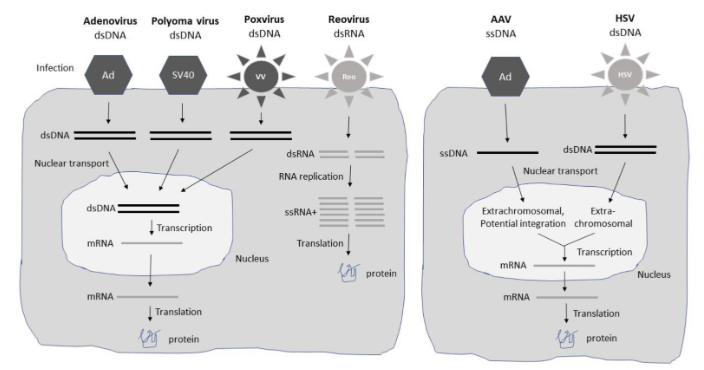 Fig.1 Expression systems for different viral vectors.1
Fig.1 Expression systems for different viral vectors.1
Our RG Virus Manufacturing for CAR-T cell therapy service offers a comprehensive solution for researchers and companies looking to produce high-quality viral vectors essential for gene delivery and cell modification. Utilizing state-of-the-art facilities and cutting-edge technologies, we ensure the production of replication-incompetent lentiviruses and other viral vectors that meet stringent regulatory and quality standards. Our team of experienced scientists provides tailored services, from the initial design phase through to the final product, guaranteeing robust titers and purity levels that enhance the efficiency of your CAR-T research. With a commitment to transparency and collaboration, we facilitate every step of the process, enabling clients to focus on their innovative work while we handle the complexities of virus manufacturing.
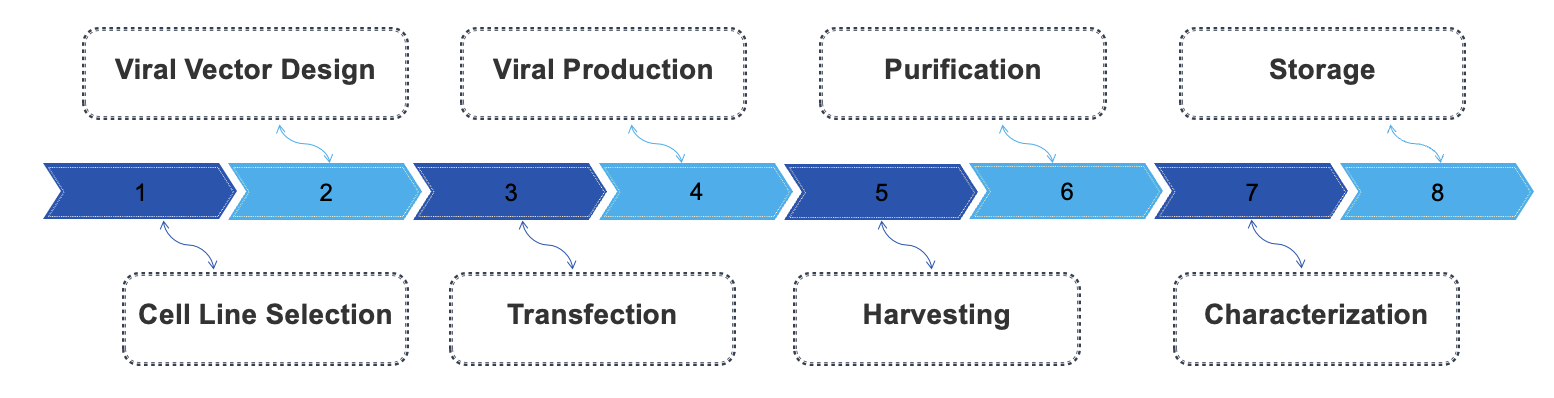
Our manufacturing process of RG virus for CAR T-cell therapy involves several meticulously controlled steps to ensure the production of high-quality viral vectors, essential for effective gene transfer. Initially, the process begins with the selection of appropriate plasmids that encode the desired CAR and any necessary viral packaging components. The plasmids are then transfected into a suitable producer cell line, often HEK293 cells, using calcium phosphate or lipid-based transfection methods. Following transfection, the cells are cultured in bioreactors under optimized conditions to facilitate viral assembly and release. Once sufficient viral titers are achieved, the supernatant is harvested and subjected to purification processes, including filtration and chromatography, to remove impurities and isolate the RG virus. Subsequently, the purified virus is characterized through techniques such as titer determination, quality control assays, and sequencing to ensure the absence of contamination and verify the integrity of the viral genome. Finally, the RG virus is formulated for storage and distribution, ensuring compliance with regulatory standards for research use.
More Global CDMO Services for CAR Viral Vector, please click here!
Experience the Creative Biolabs Advantage - Get a Quote Today!
How does Creative Biolabs ensure RG vector safety?
We use multi-plasmid packaging systems to separate viral genes, preventing replication-competent virus (RCV) formation. Each RG batch undergoes a mandatory RCV assay before release for safety assurance.
Can you produce non-lentiviral vectors like AAV?
Yes. In addition to lentivirus and retrovirus, we offer AAV packaging for non-integrating applications such as CAR-NK or in vivo studies. Please provide your vector details for customization.
How do you ensure high functional titers?
We use optimized producer cell lines, transfection reagents, and scalable bioreactors, combined with efficient purification, to achieve higher and more consistent titers than standard lab methods.
What information is needed to start production?
Provide your finalized CAR / TCR gene sequence, chosen pseudotype or serotype, and required titer/volume. These details allow immediate initiation of plasmid preparation.
Using Creative Biolabs' Research Grade CAR-T Virus Manufacturing Service in our research has significantly improved the statistical power and reliability of our in vivo tumor models. The low variability in transduction efficiency across different batches finally allowed us to confidently distinguish true therapeutic effects. Dr. M***n, Academic Research Center.
Creative Biolabs' RG service facilitated high-yield T-cell transduction, minimizing the number of primary cells required for our lead optimization studies. This advantage greatly reduced overall costs compared to previous methods, which struggled to reach 40% transduction. Dr. S***a, Oncology Biotech.
Ready to elevate your CAR-T therapy projects with our expert virus manufacturing services? Creative Biolabs' committed team is ready to assist you! For any inquiries, customized quotes, or to discuss your specific project needs, please contact us today! By partnering with us, you can expect reliable, scalable, and customized solutions to meet your specific research and clinical needs, empowering the next generation of breakthrough treatments for cancer and other diseases.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION