Cancer is a leading cause of death worldwide, highlighting the urgent need for effective treatment strategies. The adoptive transfer of tumor-infiltrating lymphocytes (TILs) is a promising method in immunotherapy that has demonstrated clinical safety and effectiveness in many trials. However, the success of TIL infusion in generating an anti-tumor response is restricted to a limited group of cancer patients. Consequently, there is an immediate need for innovative methods to improve the effectiveness of TIL-based therapies.
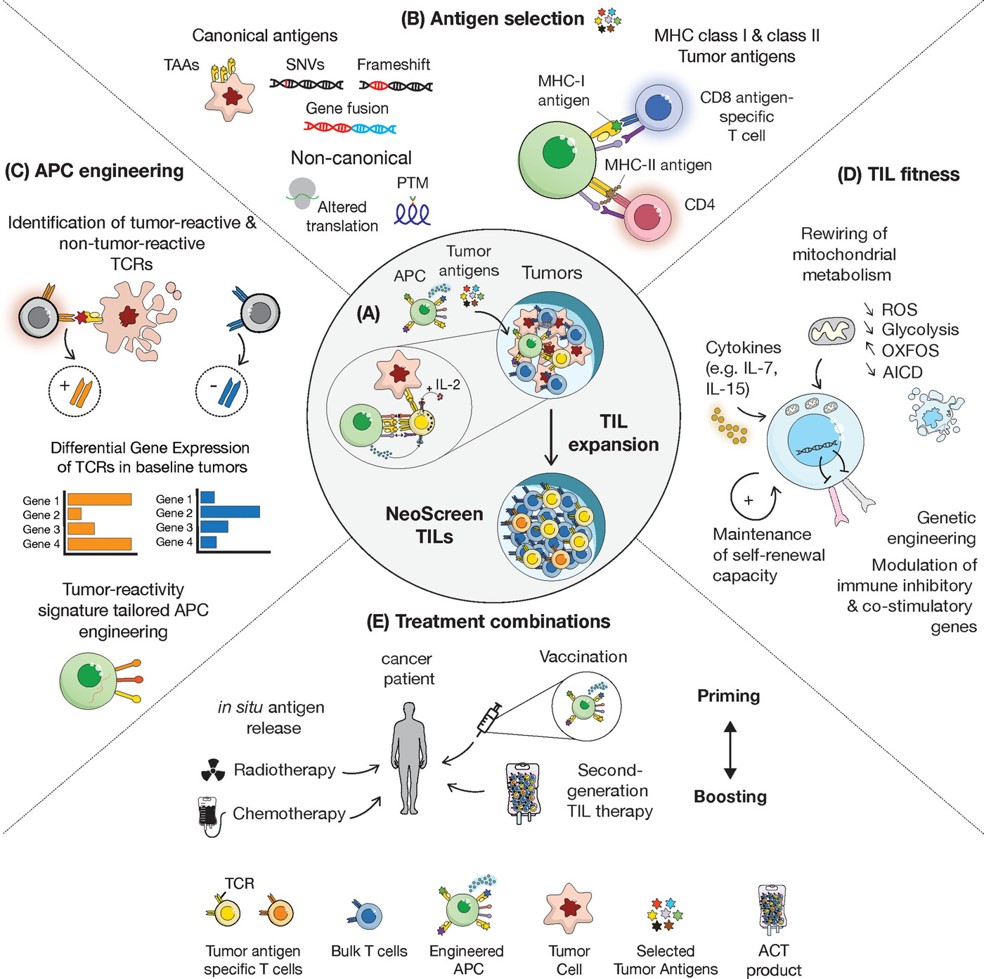 Fig.1 Overview of next-generation TIL therapy.1
Fig.1 Overview of next-generation TIL therapy.1
Creative Biolabs provides an innovative sensing system based next-generation TIL development service. By harnessing advanced genetic engineering techniques, we are able to meticulously engineer TIL cells with sophisticated sensor systems that allow for the real-time monitoring of these immune cells as they navigate through the complex environment of the body. This cutting-edge approach not only facilitates the identification and selection of TIL cells exhibiting heightened anti-tumor activity but also plays a crucial role in optimizing treatment strategies tailored to individual patient needs. Throughout the process, we strictly comply with regulatory requirements and enable rigorous quality control. Backed up by our experienced scientists, we are committed to delivering satisfactory results in customers' desired times.
Our sensing system based next-generation TIL development service covers the whole development process from the initial to validation. In addition, we also take advantage of our experience and expertise on TIL research to support global customers in preclinical and clinical studies. Listed below is our service package, including:
Sensing systems play a crucial role in optimizing TIL development and therapy. Here we provide several key types of sensing systems that can be integrated into next-generation TIL development:
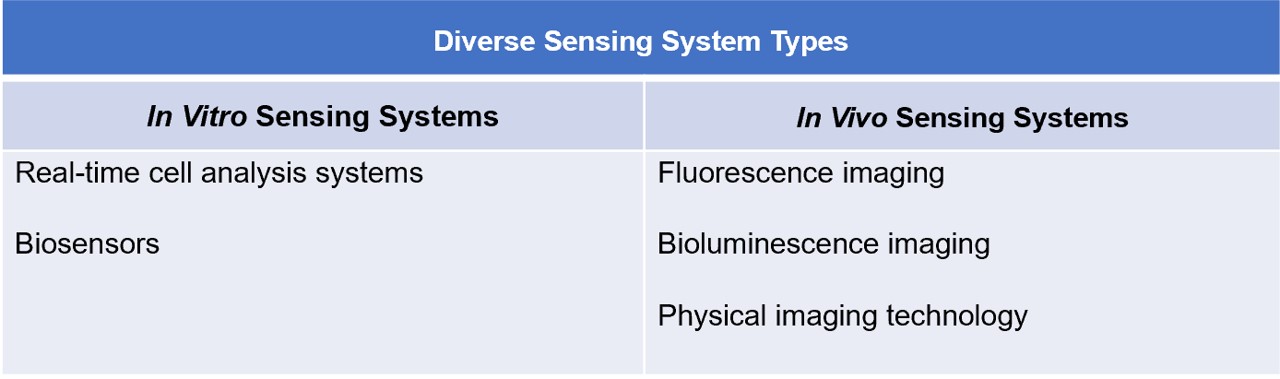 Fig.2 Diverse sensing system types.
Fig.2 Diverse sensing system types.
Q1: What process does the service follow?
A1: Our service encompasses the whole process including tumor tissue collection, TIL isolation and expansion, gene editing and modification, quality control and evaluation, and refusion. We carefully monitor and adhere to each stage to ensure that the finished product is dependable and operates effectively.
Q2: What is the difference between TIL and CAR T?
A2: TIL therapy differs from CAR T-cell therapies primarily in the source of the T cells. In CAR T-cell therapies, T cells are harvested from the patient's bloodstream. In contrast, TIL therapy involves isolating T cells directly from the patient's tumor tissue, utilizing these immune cells that have already engaged with the cancer cells in the tumor microenvironment.
If you want to know more about our sensing system based next-generation TIL development service, please feel free to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION