Each new vaccine is required to meet the strict standards issued by the European Medicines Agency (EMA) in order to be licensed. Creative Biolabs's primary serological assay allows our laboratory to test multiple strains of a single pathogen in combination with system and sample suitability controls to increase sensitivity and accuracy. Serological techniques commonly used for quantification include hemagglutination inhibition (HI), single radial hemolysis (SRH) and virus neutralization (VN) assays.
Virus Neutralization (VN) is a useful diagnostic and basic research analysis that enables observation of humoral immune responses to viral vaccines. Compared to HI, the VN assay identifies multiple neutralizing antibodies, while HI only detects antibodies to viral hemagglutinin, which acts by preventing red blood cell agglutination.
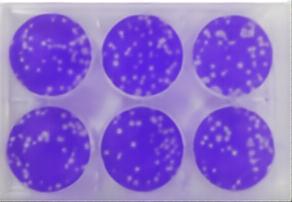
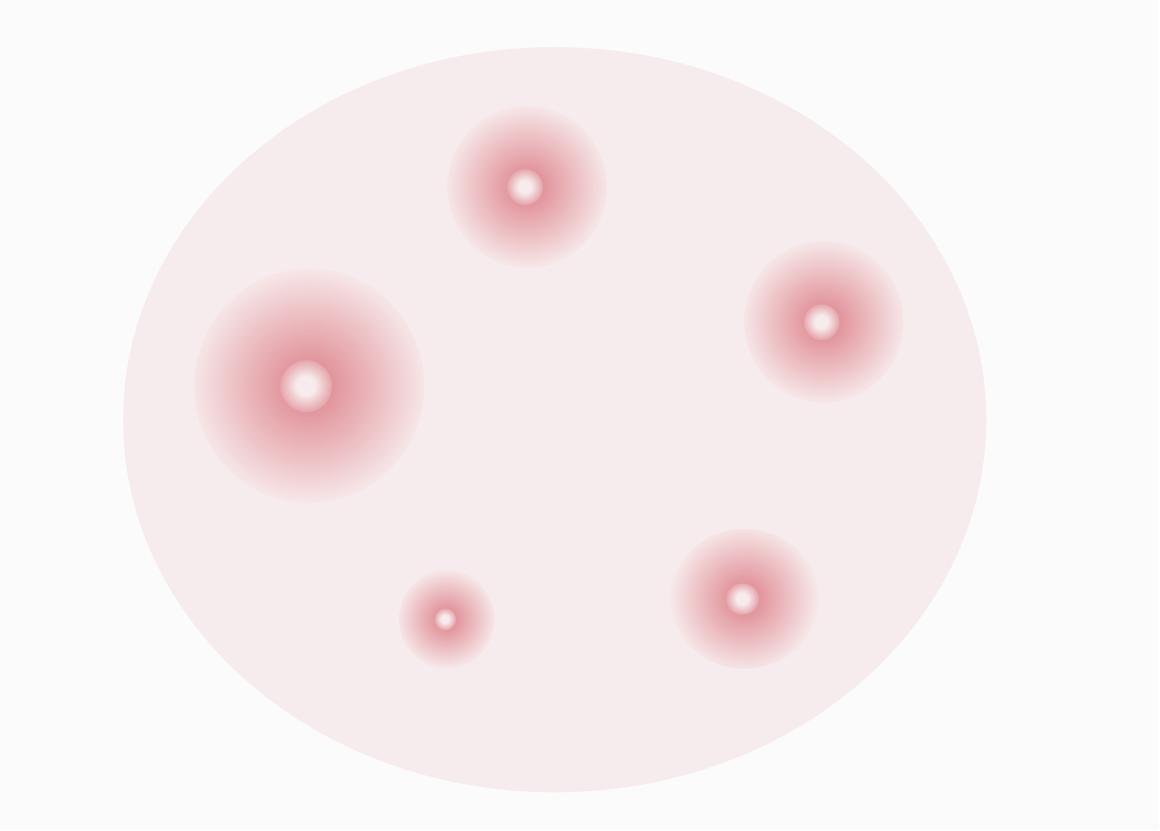
Single tibial hemolysis (SRH) combines the advantages of single radial diffusion (SRD) and HI testing. This technique utilizes antibody diffusion within the gel to determine antibodies that may present in the serum of the assay. Hemolysis mediated by complement and induced by antibody-antigen complexes produces an easily identifiable "hemolysis zone" whose size is proportional to the concentration of anti-influenza antibodies present in serum.
For more details, please feel free to contact us for project quotations and more detailed information.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION