The therapeutic landscape for solid tumors remains challenging, with many patients experiencing relapse or refractory disease despite multimodal treatments. While CAR T-cells have demonstrated unprecedented efficacy in blood cancers, their application in solid tumors encounters several significant obstacles, such as on-target, off-tumor toxicity (OTOT), antigen heterogeneity, immunosuppressive tumor microenvironment (TME), T-cell exhaustion and limited persistence. Addressing these multifaceted challenges requires innovative CAR T-cell designs that incorporate sophisticated control mechanisms and enhanced functional attributes. Creative Biolabs is committed to offering an advanced Smarter™ Sonogenetic CART development solution to address these hurdles.
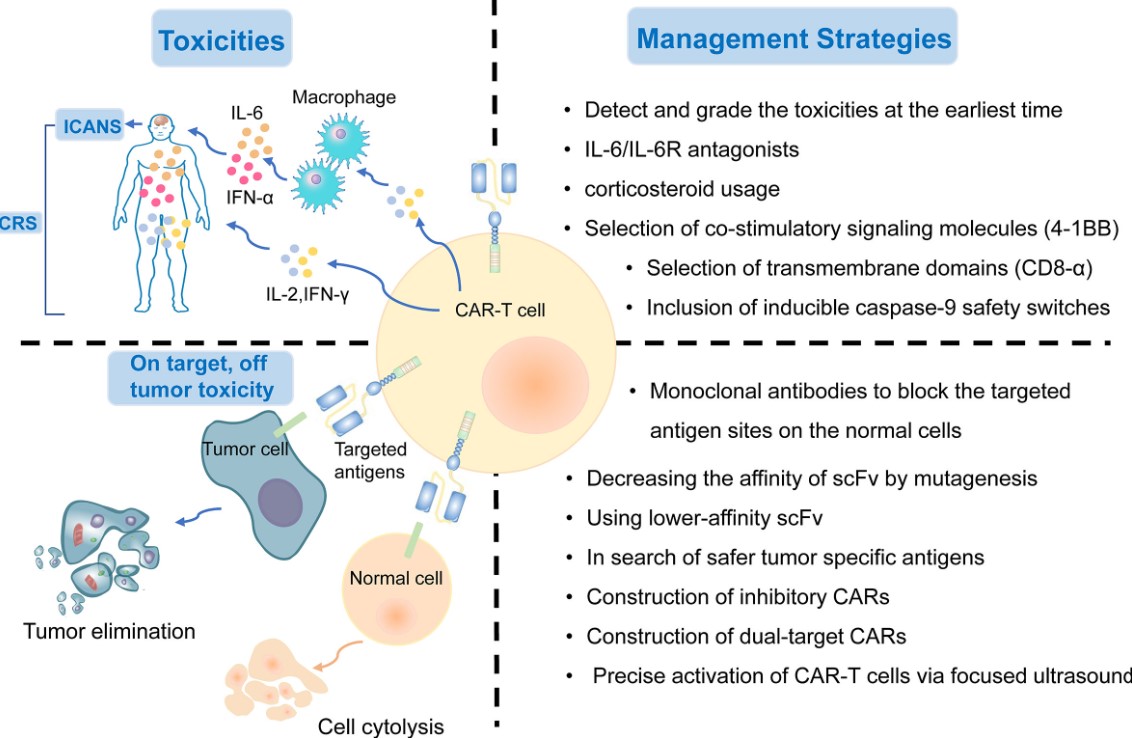 Fig.1 The on-target, off-tumor toxicities of CAR-T cell therapy.1
Fig.1 The on-target, off-tumor toxicities of CAR-T cell therapy.1
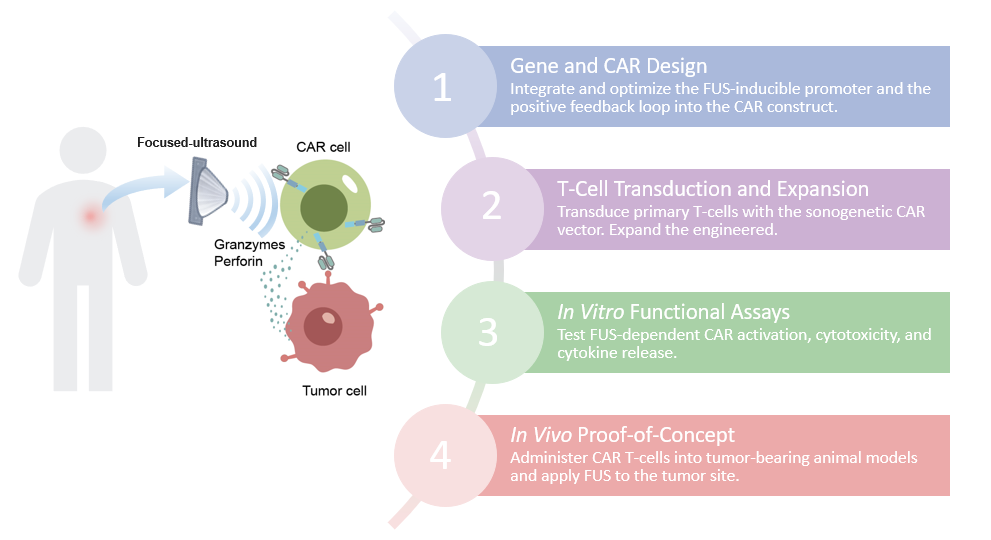
Creative Biolabs offers an integrated, end-to-end service platform for the Smarter™ Sonogenetic CART development, a cutting-edge therapeutic approach designed to overcome the limitations of conventional CAR T-cell therapy in solid tumors. This technology leverage focused ultrasound for precise spatiotemporal control of CAR expression and incorporates a novel positive feedback mechanism to sustain anti-tumor activity.
Smarter™ Sonogenetic CAR T-cells are engineered with an ultrasensitive heat-shock promoter for optimal focused ultrasound inducibility. This promoter drives CAR expression only when locally stimulated by non-invasive focused ultrasound. This "on-demand" activation ensures that the potent cytotoxic activity of the CAR T-cells is confined to the tumor site, significantly minimizing the risk of on-target, off-tumor toxicity to healthy tissues.
A key innovation is the integration of a positive feedback loop within the CAR signaling pathway. Upon initial focused ultrasound triggered CAR expression and subsequent engagement with the tumor antigen, the CAR signaling cascade itself further upregulates CAR production. This self-sustaining mechanism ensures robust and prolonged CAR expression and effector function specifically within the tumor microenvironment, even after the initial focused ultrasound stimulus is removed. This leads to enhanced cytotoxicity and persistence.
The Smarter Sonogenetic CAR-T Development Service is a comprehensive platform designed to create next-generation, precision-guided CAR-T cells, primarily for solid tumors. The core innovation is using non-invasive focused ultrasound to remotely and locally activate the CAR T-cells. Here is a breakdown of the services and the analyses provided:

Kindly reach out to us to schedule a private consultation and obtain a detailed technical proposal.
Creative Biolabs provides a tailored suite of services specifically designed to support the unique requirements of Smarter™ Sonogenetic CAR T-cell development. Our comprehensive offerings span the entire preclinical development pipeline:
Q1: What fundamentally distinguishes sonogenetic Smarter™ Sonogenetic CART-cells from standard CAR T-cells in the context of solid tumors?
A1: Smarter™ Sonogenetic -CAR T-cells offer two primary distinctions: 1) Spatiotemporal Control: Their activity is triggered by focused ultrasound specifically at the tumor site, drastically reducing systemic exposure and on-target, off-tumor toxicity. 2) Sustained Activity: An engineered positive feedback loop ensures that once activated, tumor engagement leads to prolonged CAR expression and effector function, combating T-cell exhaustion and improving persistence.
Q2: What are the principal safety advantages offered by the Smarter™ Sonogenetic CART-cell approach?
A2: The primary safety advantage is the significant reduction in on-target, off-tumor toxicity. By restricting CAR expression and activity to the focused ultrasound-targeted tumor area, systemic exposure of healthy tissues expressing the target antigen at low levels is minimized.
Q3: How does Creative Biolabs ensure the quality and reproducibility of Smarter™ Sonogenetic CART development projects?
A3: Creative Biolabs employs rigorous quality control measures at every stage of development. This includes validated assays, standardized operating procedures, meticulous record-keeping, and the use of well-characterized reagents and models. Our scientific team comprises experts with extensive experience in cell therapy development.
Q4: What is the anticipated development timeline for the Smarter™ Sonogenetic CART therapy when partnering with Creative Biolabs?
A4: The timeline can vary depending on the project's specific scope, target complexity, and starting point. However, Creative Biolabs' integrated "one-stop" service model is designed to optimize efficiency and reduce overall development timelines compared to managing multiple disparate service providers.
Creative Biolabs is dedicated to advancing the next generation of cancer immunotherapies. If you are developing or considering a sonogenetic CAR T-cell program, or wish to explore how the Smarter™ Sonogenetic -CAR T platform can transform your solid tumor therapeutic strategy, we invite you to connect with our experts. Partner with Creative Biolabs to harness the power of precision-guided, sonogenetically controlled CAR T-cell therapies and bring hope to patients with challenging solid tumors.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION