Tumor-infiltrating lymphocyte (TIL) therapy is one possible approach to treat solid tumors. By harnessing the body's immune system, TIL therapy offers the potential to target and eliminate cancer cells. Moreover, synthetic receptors are expected to improve TIL therapy and overcome the challenges of traditional TIL therapy. These engineered proteins can be designed to recognize specific tumor antigens, increasing the targeting precision and potency of TIL cells. This method has the potential to significantly enhance patient outcomes and alter cancer therapy.
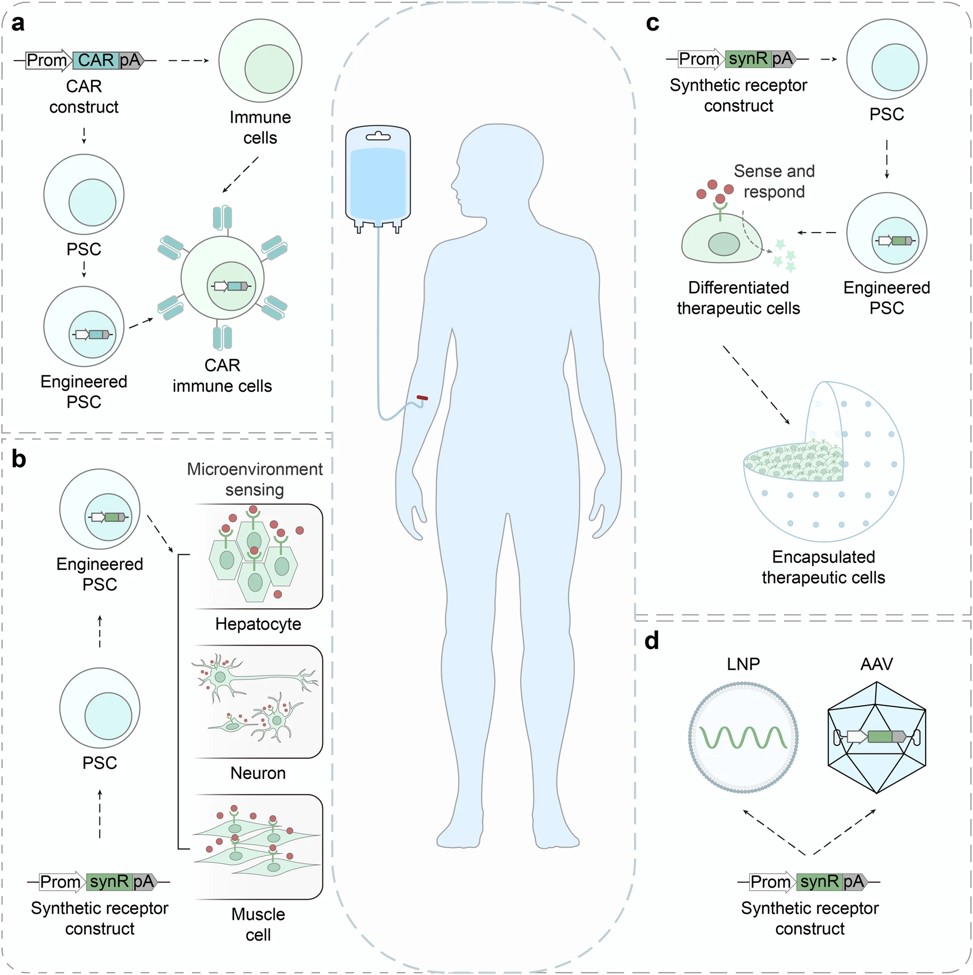 Fig.1 Synthetic receptors' growing range of possible therapeutic applications.1
Fig.1 Synthetic receptors' growing range of possible therapeutic applications.1
Synthetic receptor-based next-generation TIL therapy is a promising frontier in cancer immunotherapy. As a leader in cancer research, Creative Biolabs offers a comprehensive service to enhance the potency and specificity of TIL therapies. Our innovative approach addresses the limitations of traditional TIL therapies, such as low numbers of isolated TIL, TIL exhaustion, and the immunosuppressive tumor microenvironment.
Our synthetic receptor based next-generation TIL development service encompasses a range of critical steps, which include: synthetic receptor design and engineering, TIL isolation and expansion, synthetic receptor transduction, functional characterization and quality control, as well as preclinical and clinical development support. Our service covers the whole process (Listed below) to provide more convenience for global customers. In addition, we employ cutting-edge technologies and experienced scientists to ensure the highest quality and efficacy of engineered TILs. By employing our extensive expertise, we enable worldwide customers to engineer breakthrough TIL therapeutics that have the potential to enhance patient outcomes and transform cancer therapy.
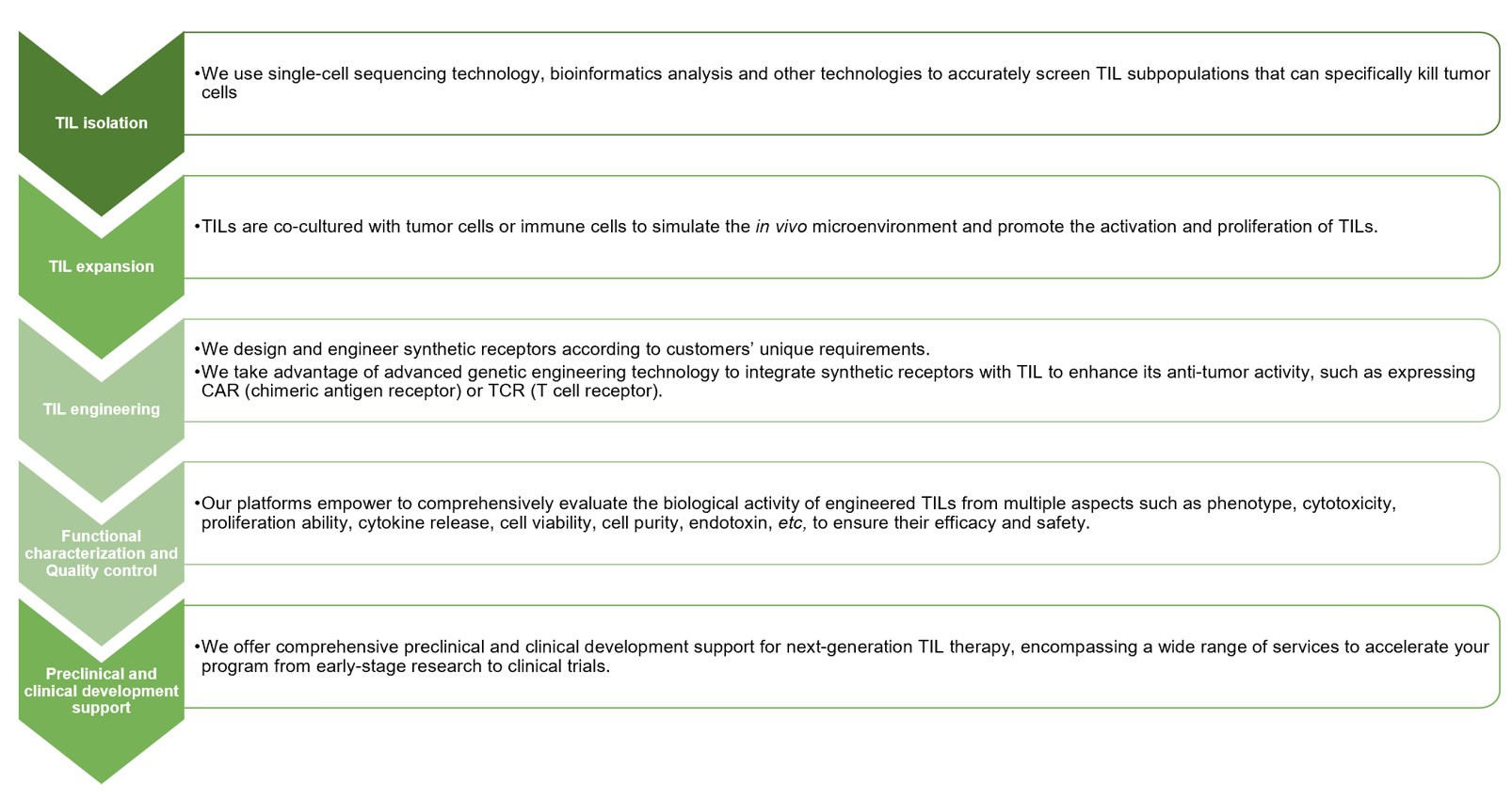 Fig.2 Our comprehensive synthetic receptor based next-generation TIL development service process.
Fig.2 Our comprehensive synthetic receptor based next-generation TIL development service process.
Synthetic receptors can be categorized into cell-surface and intracellular receptors based on their ligand-binding location. Leveraging our robust platform, Creative Biolabs has the ability to synthesize and engineer a wide range of synthetic receptors to cater to diverse research requirements, including but not limited to the following:
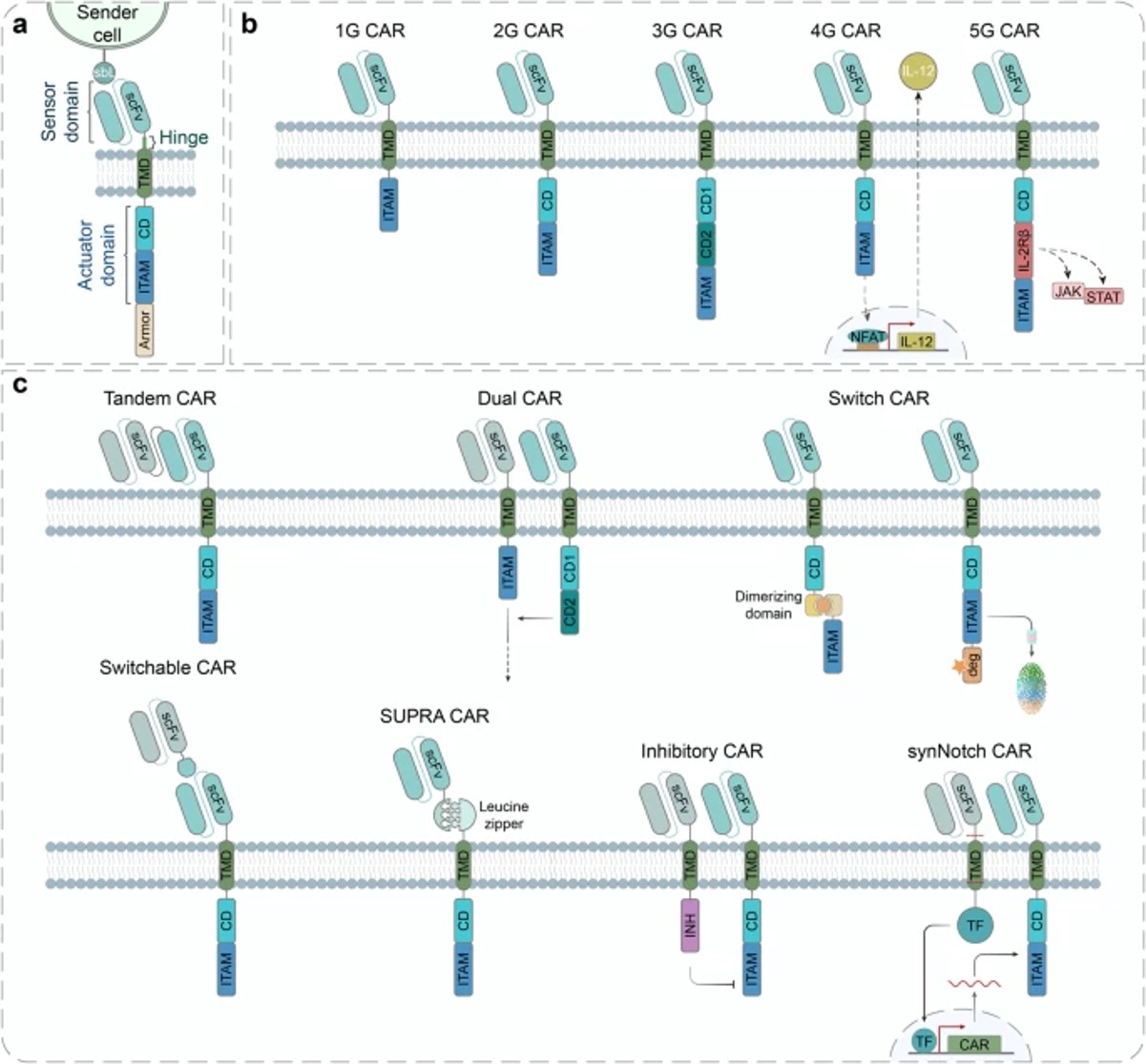 Fig.3 The chimeric antigen receptor's (CAR) design and engineering.1
Fig.3 The chimeric antigen receptor's (CAR) design and engineering.1
Q1: How can Creative Biolabs guarantee the safety and effectiveness of engineered TILs?
A1: Creative Biolabs adheres to rigorous quality control standards throughout the development process. This includes careful selection of donor cells, optimization of culture conditions, and rigorous testing of engineered TILs for potency, specificity, and safety.
Q2: What is the timeline for developing synthetic receptor-engineered TIL therapies?
A2: The timeline for developing synthetic receptor-engineered TIL therapies can vary depending on factors such as the complexity of the synthetic receptor design, the regulatory requirements, and the specific clinical trial design. However, Creative Biolabs is committed to accelerating the development process to bring these therapies to patients as quickly as possible.
Q3: What is the cost of Creative Biolabs' synthetic receptor-based TIL development service?
A3: The cost of the service can vary depending on the specific project requirements, such as the complexity of the synthetic receptor design, the number of TILs required, and the level of preclinical and clinical development support needed. Please contact Creative Biolabs for a detailed quote.
For more detailed information about our synthetic receptor based next-generation TIL development service, please don't hesitate to Get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION