T cell-mediated tumor cell lysis represents a critical effector mechanism in adaptive antitumor immunity, enabling specific elimination of malignant cells through perforin-granzyme and death receptor pathways. Creative Biolabs' T Cell-mediated Tumor Cell Lysis Assay Service provides quantitative, reproducible analysis of cytotoxic activity for a wide range of effector and target cell systems, including patient-derived samples and engineered T cell products. This service delivers essential functional data to accelerate candidate selection, optimize therapeutic efficacy, and de-risk translation into preclinical and clinical applications.
T cell-mediated tumor cell lysis is a process by which cytotoxic T lymphocytes (CTLs) identify and eliminate malignant cells through direct intercellular contact, primarily via the perforin-granzyme pathway and death receptor signaling. Understanding this mechanism is essential for evaluating adaptive antitumor immunity and for developing T cell-based immunotherapies, such as CAR-T and TCR-T therapies. Therefore, robust and quantitative T cell-mediated tumor cell lysis assays are of significant importance for assessing CTL efficacy, screening novel therapeutic agents, and predicting clinical responses in cancer immunotherapy.
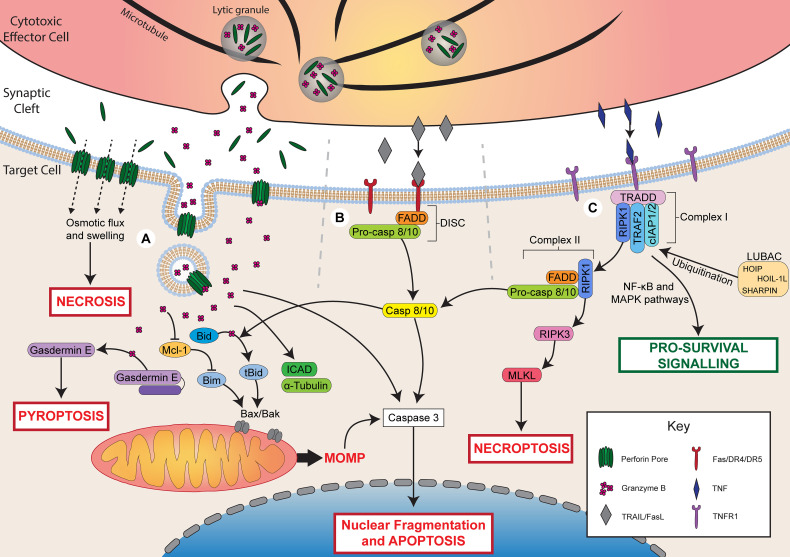 Fig.1 Effector cell-mediated target cell killing mechanisms.1
Fig.1 Effector cell-mediated target cell killing mechanisms.1
Creative Biolabs' T Cell-mediated Tumor Cell Lysis Assay Service leverages the antigen-specific cytolytic activity of engineered T cells against tumor cells to quantitatively assess their killing efficiency. Through rigorous and reproducible assays, we help accelerate the transition from research to clinical application by confirming the functional integrity and therapeutic potential of cell-based immunotherapies.
Our service offers a flexible and robust platform to support your immunotherapy development. We provide two distinct assay formats to accurately quantify T cell-induced tumor cell killing, enabling precise evaluation of cytotoxic efficacy in both research and preclinical settings.
This assay utilizes radiolabeled isotopes that are incorporated into the cytosolic proteins of target tumor cells, enabling quantitative detection of released radioactivity upon CAR-T cell-mediated killing. Its key advantage lies in the high sensitivity and reproducibility of radiolabel-based measurement, making it particularly suitable for high-throughput screening and dose-response studies.
This method employs fluorescent cytoplasmic dyes to label tumor cells, allowing real-time, live-cell imaging to dynamically monitor CAR-T‐induced cytolysis. A significant advantage of this system is its ability to support longitudinal tracking of cell viability and function under physiologically relevant conditions, while preserving cellular viability and enabling flexible optimization of effector-to-target ratios.
Key Steps Involved:
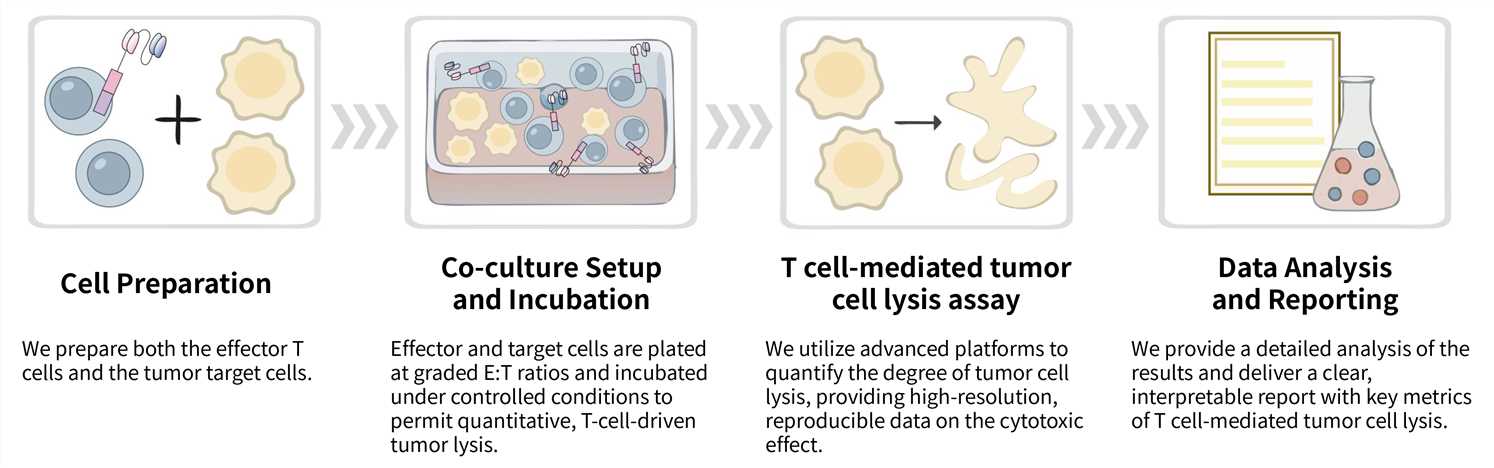
Final Deliverables:
What is the primary application of your T cell-mediated tumor cell lysis assay service?
This service is primarily used to assess the potency and efficacy of emerging immunotherapies such as CAR-T, TCR-T, bispecific antibodies, and other T-cell engagers. It delivers critical insights into the capacity of your candidate therapy to mediate specific tumor cell lysis.
Can your assays accommodate patient-derived cells or custom cell lines?
Sure, we support a broad spectrum of cellular models, including patient-derived samples, primary cell cultures, and custom-engineered tumor lines. Recognizing the importance of personalized medicine, we offer adaptable assay configurations to suit specialized research needs.
Creative Biolabs has built a strong reputation in the field, backed by extensive expertise and a steadfast commitment to high-quality scientific outcomes. Our robust assay systems are founded on well-established experimental protocols and cutting-edge technologies, ensuring both reliability and high translational value of the data.
"Employing Creative Biolabs' T Cell-mediated Tumor Cell Lysis Assay Service greatly accelerated our screening workflow and enhanced the reproducibility of our results. Their high-throughput platform enabled rapid evaluation of hundreds of candidate cells, while consistently delivering data of exceptional quality." Jn De.
"The lytic activity data proved critical in confirming the efficacy of our lead T-cell construct. Creative Biolabs provided expert technical support to optimize a challenging co-culture system, ultimately demonstrating the significant cytotoxic enhancement we aimed to achieve. The comprehensive report contained all necessary details for our regulatory filing." Aa Rs.
"After encountering considerable variability with in-house assays, we transitioned to Creative Biolabs for their standardized procedures and precise execution. Their ability to incorporate a custom cell line seamlessly addressed our specific project needs, yielding reliable and consistent data that bolstered our confidence in the outcomes." Cs Mr.
Building upon extensive research expertise in CAR technology, Creative Biolabs offers an integrated One-Stop CAR-T Therapy Development service. This end-to-end solution spans from CAR design and construction to comprehensive in vitro and in vivo functional validation of CAR-T products. For research and development support in this advancing field, we invite you to connect with our specialist team. Our scientists are readily available to assist with your project requirements.
At Creative Biolabs, we are committed to driving progress in T-cell immunotherapy through the delivery of cutting-edge research tools and specialized services. Our team of experts is ready to support your innovative projects—contact us today to discover how our solutions can accelerate your next discovery.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION