CART therapy has revolutionized the treatment of several hematological malignancies, demonstrating remarkable clinical response rates. While CAR-T cell therapies have shown remarkable clinical effect, they are associated with toxicities, including immune effector cell–associated neurotoxicity syndrome (ICANS), and cytokine release syndrome (CRS). Recent research has identified a distinct neurotoxicity syndrome, tumor inflammation-associated neurotoxicity (TIAN), particularly relevant in patients treated with cell therapies for tumors in the central nervous system (CNS).
The successful management of CAR-T cell therapy-induced toxicities, including TIAN, hinges on the ability to predict, detect, and monitor these adverse events effectively. Biomarker validation plays a crucial role in this process, providing objective measures of biological processes, disease states, or clinical effect to therapeutic interventions.
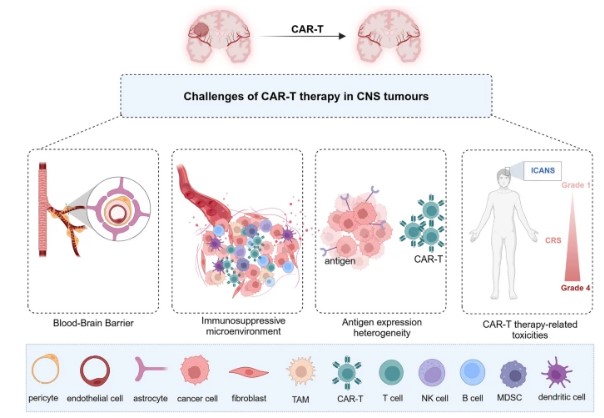 Fig.1 CAR-T therapy challenges.1
Fig.1 CAR-T therapy challenges.1
Creative Biolabs offers specialized biomarker validation services, focusing on TIAN, to support the development of effective CAR-T cell therapy toxicity management solutions. TIAN presents with unique clinical manifestations that differ from the systemic toxicities of CRS and ICANS. Effective management strategies require a deep understanding of the underlying pathophysiological mechanisms and the ability to identify patients at high risk of developing TIAN.
Our biomarker validation efforts are essential for:
Creative Biolabs' comprehensive biomarker validation service supports the entire biomarker lifecycle, from discovery to clinical application. We offer a range of platforms for biomarker quantification:
Our services encompass:
Q1: What is the benefit of TIAN related Biomarker Validation?
A1: Biomarker validation helps in predicting, diagnosing, and monitoring TIAN, improving patient outcomes and the safety of CAR-T therapies.
Q2: What is the sample needed to validate the biomarker?
A2: Creative Biolabs analyzes a variety of sample types, including blood, serum, plasma, and CSF.
Creative Biolabs is committed to partnering with pharmaceutical and biotech companies to advance the development of effective CAR-T cell therapy toxicity management solutions. For inquiries about our biomarker validation services, please contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION