The complex neurotoxicities associated with CAR T-cell therapy are broadly categorized, with cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) being the most recognized. However, an emerging and distinct entity, Tumor Inflammation-Associated Neurotoxicity (TIAN), specifically observed in patients with central nervous system (CNS) tumors undergoing immunotherapies, highlights the nuanced landscape of these adverse events. Unlike systemic toxicities, TIAN's manifestations are often localized, reflecting the tumor's neuroanatomical position. The pathophysiology of these neurotoxicities is multifactorial, driven by the systemic inflammatory response, often termed a "cytokine storm," involving a cascade of inflammatory mediators such as IL-6, TNF-α, and IFN-γ. This inflammatory milieu can lead to endothelial activation, disruption of the blood-brain barrier, and direct neurotoxicity.
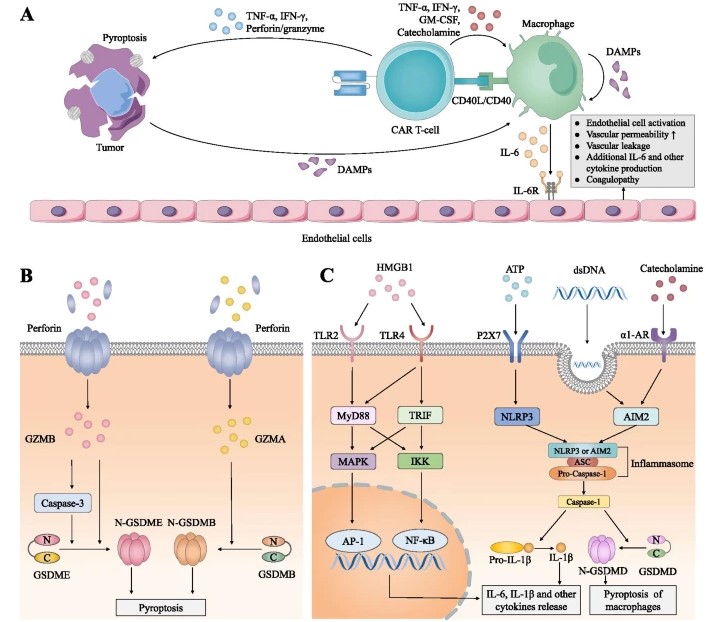 Fig.1 CRS toxicity related to CAR T-cell therapy.1
Fig.1 CRS toxicity related to CAR T-cell therapy.1
At Creative Biolabs, with over two decades of specialized experience in biological research and immune system analysis, we recognize that a profound understanding of the immune system's response to CAR T-cell therapy is paramount for effective toxicity management. Our dedicated TIAN-related Immune System Study Service, leveraging advanced immunodeficient models, is designed to unravel these complex immunological mechanisms, providing actionable insights for safer and more effective therapeutic interventions. These models are critical for studying human immune cell behavior and CAR T-cell interactions in vivo without confounding host immune responses, enabling precise investigation of TIAN pathophysiology and the evaluation of novel mitigation strategies. We are committed to supporting our clients in navigating the challenges of CAR T-cell-induced toxicities, from early-stage drug discovery to late-phase clinical trials.
Creative Biolabs offers a comprehensive suite of services tailored to investigate the immune system's role in CAR T-cell toxicities, including TIAN, CRS, and ICANS, within the controlled environment of immunodeficient models. Our approach integrates cutting-edge technologies with expert biological interpretation to provide a holistic view of immune dysregulation.
Our capabilities extend to high-resolution immune phenotyping using multi-parameter flow cytometry and mass cytometry, specifically adapted for analysis of human immune cells engrafted within immunodeficient models. These platforms enable the precise characterization of human immune cell populations (T-cells, B-cells, myeloid cells, NK cells) and their activation states, exhaustion markers, and differentiation profiles in vivo. By meticulously analyzing these cellular dynamics, we can identify specific immune cell subsets or activation patterns that correlate with the onset and severity of neurotoxicities, serving as crucial predictive or prognostic biomarkers for TIAN, CRS, and ICANS in a controlled experimental setting. This deep cellular profiling is essential for understanding the cellular drivers behind the inflammatory responses.
The "cytokine storm" is a hallmark of severe CAR T-cell toxicities. Creative Biolabs employs highly sensitive multiplex assays, such as Multiplex bead-based immunoassay and ELISA, to quantify a broad panel of inflammatory cytokines and chemokines (e.g., IL-6, IL-1, TNF-α, IFN-γ, IL-10, MCP-1, IP-10) in biological samples (e.g., serum, plasma, CSF) derived from immunodeficient models. Monitoring these soluble mediators provides critical insights into the intensity and progression of the systemic inflammatory response that underpins CRS and neuroinflammation, including ICANS and TIAN, within a controlled experimental system. Our robust methodologies ensure accurate and reproducible quantification, vital for mechanistic studies and preclinical evaluation of therapeutic interventions.
Beyond mere quantification, understanding the functional capacity of immune cells is crucial. Creative Biolabs performs a range of functional immune assays, including T-cell activation, proliferation, and cytotoxicity assays, specifically adapted for human cells within immunodeficient models. These studies assess the effector function of CAR T-cells and other human immune cells, helping to elucidate how their activity contributes to both therapeutic efficacy and adverse events. Furthermore, we investigate myeloid cell function, as these cells play a significant role in mediating inflammatory responses. By studying these functional aspects in a controlled model, we can gain deeper insights into the mechanisms driving immune effector cell-associated toxicities and evaluate the impact of potential mitigating agents.
For neurotoxicities like TIAN and ICANS, direct assessment of CNS inflammation is paramount. Creative Biolabs offers specialized assays for biomarkers indicative of neuronal damage and neuroinflammation in cerebrospinal fluid (CSF) and serum obtained from immunodeficient models. These include glial fibrillary acidic protein (GFAP), neurofilament light chain (NFL), and specific CSF cytokine profiling. Such targeted analyses provide direct evidence of CNS involvement and help differentiate between various forms of neurotoxicity in a controlled experimental setting, supporting precise mechanistic understanding and the preclinical evaluation of tailored management strategies.
Creative Biolabs' expertise extends to monitoring pharmacodynamic biomarkers throughout preclinical studies utilizing immunodeficient models, allowing for real-time assessment of drug activity and early detection of potential toxicities. Our integrated approach supports translational research, bridging the gap between in vitro findings and clinical application. We assist clients in designing studies, analyzing data, and interpreting results to accelerate the development of safer and more effective immunotherapies.
Creative Biolabs stands as a trusted partner in the complex field of CAR T-cell therapy development due to several key advantages:
Q1: What is the primary difference between CRS, ICANS, and TIAN?
A1: Cytokine Release Syndrome (CRS) is a systemic inflammatory response characterized by fever, hypotension, and hypoxia, driven by widespread cytokine release. Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) is a broader neurotoxicity syndrome that can occur after CAR T-cell therapy, presenting with diverse neurological symptoms like encephalopathy, seizures, and aphasia. Tumor Inflammation-Associated Neurotoxicity (TIAN) is a distinct neurotoxicity syndrome observed specifically in patients with CNS tumors treated with immunotherapies, where inflammation at the tumor site leads to localized neurological symptoms, differentiating it from the more generalized ICANS.
Q2: How can immune system studies using immunodeficient models help manage CAR T-cell toxicities?
A2: Immune system studies in immunodeficient models provide critical insights into the underlying mechanisms of CAR T-cell toxicities in a controlled in vivo environment. By allowing for the precise engraftment of human immune cells and tumors, these models enable the identification of specific immune cell subsets, cytokine profiles, and neuroinflammation markers that drive toxicities. This helps predict toxicity onset, monitor severity, and evaluate the efficacy of novel therapeutic interventions for TIAN, CRS, and ICANS in a preclinical setting, ultimately leading to the development of safer and more targeted management strategies for clinical translation.
Q3: What types of samples does Creative Biolabs analyze for CAR T-cell toxicity studies in immunodeficient models?
A3: Creative Biolabs routinely analyzes a variety of biological samples derived from immunodeficient models, including human peripheral blood mononuclear cells (PBMCs) from engrafted animals, serum, plasma, and cerebrospinal fluid (CSF), depending on the specific research question and the nature of the toxicity being investigated. We also analyze tumor tissue and brain tissue for localized inflammatory responses.
Creative Biolabs is your dedicated partner in advancing the field of cancer immunotherapy by providing unparalleled expertise in TIAN-related immune system studies and comprehensive CAR T-cell toxicity management solutions, specifically leveraging the power of immunodeficient models. Let us help you accelerate your research, enhance patient safety, and bring life-changing therapies to those who need them most.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION