The emergence of adoptive cellular therapies (ACTs) as a potent treatment for cancer has driven the necessity to discover tumor-reactive T cell receptors (TCRs) that can precisely target neoplastic cells. With the cancer burden increasing globally, the development of targeted immunotherapies is not only urgent but also a pivotal advancement in oncological treatments. Traditional methods of TCR discovery are hampered by limitations related to throughput and the ability to effectively identify clinically relevant TCRs that recognize patient-specific neoantigens. This scenario underscores the critical need for innovative platforms capable of high-throughput and precise identification of tumor-reactive TCRs.
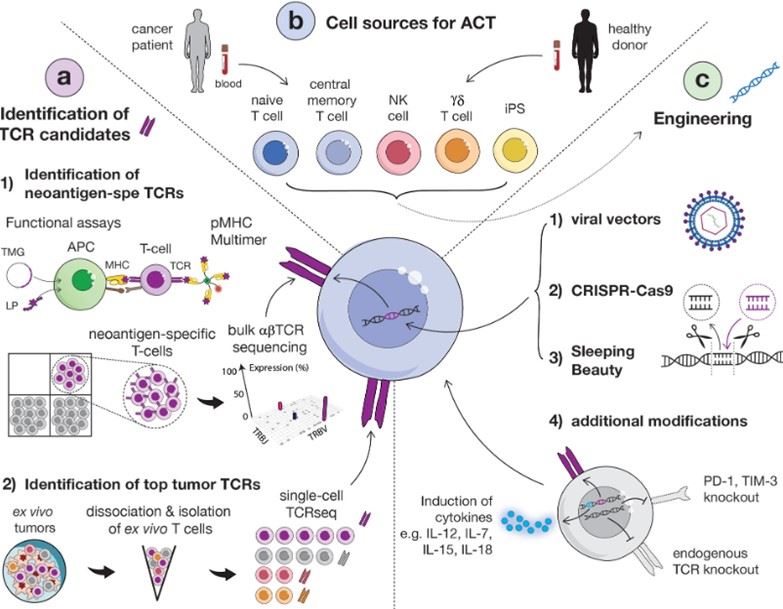 Fig.1 Overview diagram of the main strategies for identifying relevant TCRs.1
Fig.1 Overview diagram of the main strategies for identifying relevant TCRs.1
Creative Biolabs, leveraging cutting-edge biotechnological tools, has developed a high-throughput, personalized TCR discovery pipeline. This platform is designed to address the scarcity of tumor-specific T cells within patient repertoires and the patient-specific nature of tumor epitopes. It facilitates the assembly of complex synthetic TCR libraries effortlessly through a one-pot reaction. The subsequent pooled expression in reporter T cells allows functional genetic screening against patient-derived tumor or antigen-presenting cells.
The identified TCRs have substantial potential in clinical translation. For example, virus-specific TCRs such as EBV and CMV can be identified through the platform to restore viral immunity after hematopoietic stem cell transplantation.
With methodologies removing substantial bottlenecks in functional TCR discovery, Creative Biolabs' platform can facilitate novel ACT development. This advancement is crucial for expediting the journey from bench to bedside, specifically in harnessing TCRs that are not artificially affinity-matured, thus potentially reducing off-target toxicities.
Q1: Why is high-throughput TCR discovery critical in cancer therapy?
A1: High-throughput TCR discovery enables the efficient identification of numerous TCRs that can target a wide array of tumor antigens, enhancing the precision and efficacy of immunotherapies.
Q2: How does the platform address the issue of false positives in TCR identification?
A2: The combined use of activation-based screening with dextramer binding substantially lowers the rate of false positives, ensuring that only functionally relevant TCRs are identified.
Q3: What are the clinical implications of this TCR discovery platform?
A3: The platform's identified TCRs have implications in developing novel ACTs, potentially offering more effective and less toxic therapeutic options for cancer and viral infections.
Creative Biolabs has curated a state-of-the-art platform that encompasses robust and efficient TCR discovery, poised to transform the landscape of adoptive cellular therapies and personalized medicine in oncology and beyond.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION