Hepatic fibrosis is a major worldwide health concern, causing severe liver dysfunction and end-stage liver disease. Creative Biolabs is pioneering novel therapeutic avenues with our anti-uPAR CAR macrophages development service for hepatic fibrosis, engineered to precision-target fibrotic pathways. Our innovative service helps you accelerate therapeutic development and obtain high-quality, specifically engineered cell therapies by leveraging advanced genetic engineering and cell-based immunotherapy techniques for the precise resolution of fibrotic pathology.
Excessive extracellular matrix formation is a hallmark of hepatic fibrosis, which is a leading cause of liver failure worldwide. Current therapeutic options are largely palliative, highlighting the urgent need for targeted, curative interventions. Gene therapy, particularly cell-based approaches like engineered macrophages, offers immense promise. As indicated by recent research, understanding the role of key receptors such as uPAR in fibrogenesis is crucial for developing precise treatments. The development of anti-uPAR CAR macrophages directly addresses this unmet need by taking advantage of macrophages' innate phagocytic and immunomodulatory properties to target and clear uPAR-expressing fibrogenic cells and pathological components, providing a novel and highly specific therapeutic strategy for fibrosis reversal.
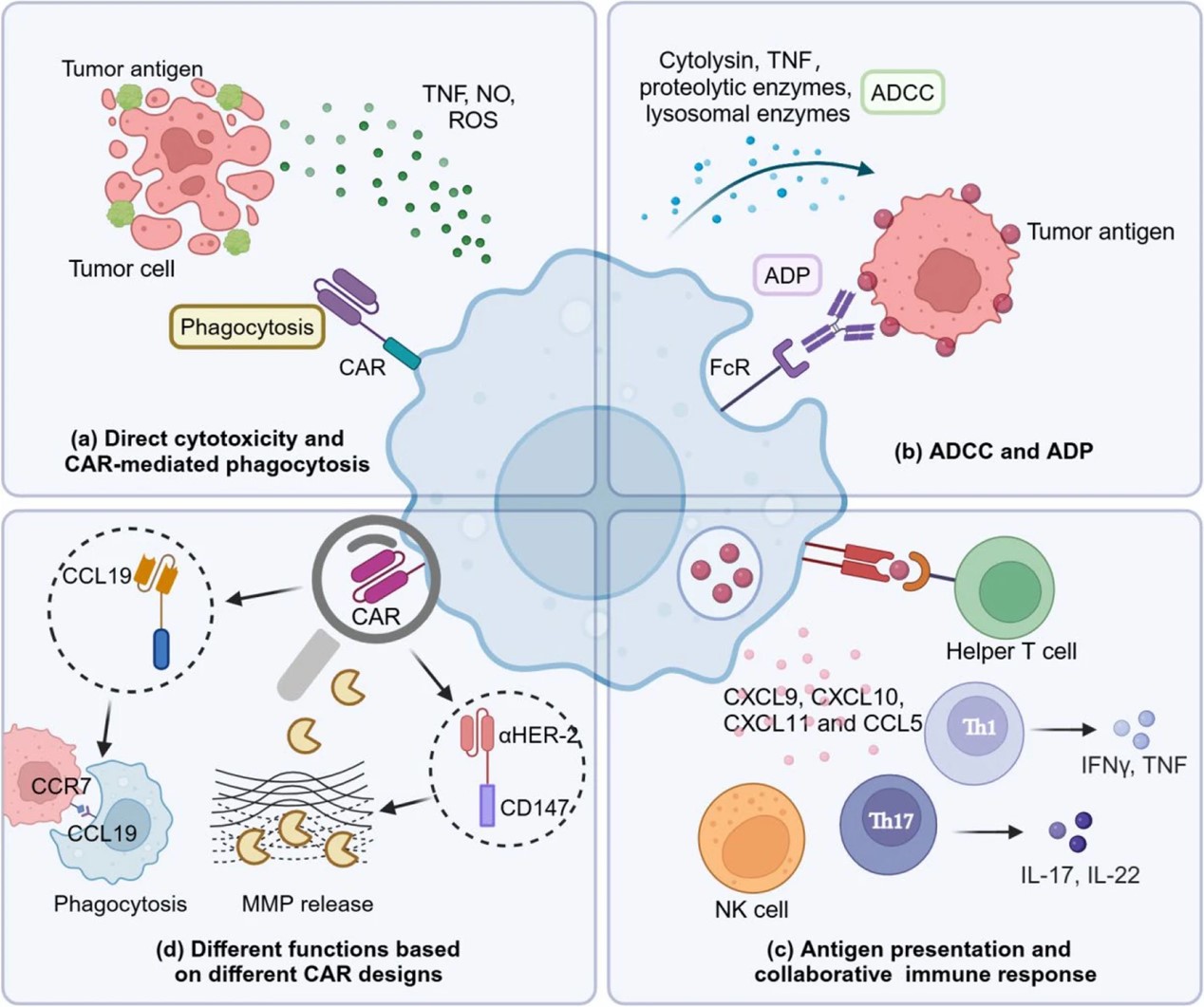 Fig.1 CAR macrophage therapies.1
Fig.1 CAR macrophage therapies.1
At Creative Biolabs, our anti-uPAR CAR macrophages development service provides comprehensive solutions, meticulously tailored to your specific research and therapeutic goals in combating hepatic fibrosis. Leveraging advanced CAR engineering, we develop precisely targeted macrophage cell products that operate on the principle of specific recognition and phagocytosis of uPAR-expressing fibrogenic cells, coupled with the inherent immunomodulatory functions of macrophages to resolve fibrosis. Our commitment to robust functional data and detailed characterization ensures the highest quality deliverables, backed by a typical project timeline ranging from 12 to 20 weeks depending on project scope. This rigorous approach is designed to instill confidence and significantly advance your preclinical and translational projects.
We begin by designing the optimal anti-uPAR CAR construct, incorporating a uPAR-specific single-chain variable fragment (scFv) linked to appropriate signaling domains (CD3ζ, co-stimulatory molecules like CD28 or 4-1BB). This design is then cloned into a suitable viral (lentivirus, retrovirus) or non-viral vector for efficient macrophage transduction.
Primary macrophages are isolated from client-provided or ethically sourced donor cells (e.g., monocytes from PBMCs) and differentiated in vitro into functional macrophage subsets. Our protocols ensure high purity and viability of macrophage populations suitable for genetic modification.
The engineered vector is then introduced into the isolated macrophages using optimized transduction protocols. Post-transduction, the CAR-expressing macrophages are carefully expanded under controlled conditions to achieve the required cell numbers for downstream applications.
Rigorous quality control and characterization are performed. This includes flow cytometry for CAR expression, assessment of macrophage phenotype (M1/M2 polarization), viability, purity, and safety assays (e.g., mycoplasma testing). Functional assays, such as uPAR-specific binding and phagocytosis assays using uPAR-positive target cells, are conducted.
For projects requiring advanced validation, we offer in vitro assays assessing CAR macrophage-mediated clearance of uPAR-expressing fibrogenic cells (e.g., activated HSCs) and extracellular matrix degradation. Furthermore, in vivo studies in relevant animal models of hepatic fibrosis can be performed to evaluate therapeutic efficacy, biodistribution, and safety.
We work to optimize the entire development process for consistency, reproducibility, and scalability, crucial for future translational and manufacturing needs.
Our clients experience an average 45% increase in targeted cell clearance efficiency and a 30% reduction in fibrotic marker expression in preclinical models.
Case One----
Through our Anti-uPAR CAR Macrophages Development Service, our client successfully identified multiple promising CAR macrophage candidates demonstrating superior in vitro phagocytic activity against uPAR-positive hepatic stellate cells within 16 weeks, significantly advancing their preclinical program.
Case Two----
After utilizing our Anti-uPAR CAR Macrophages Development Service, our client successfully demonstrated significant reduction of liver fibrosis and improved liver function in a rodent model, validating a novel therapeutic approach that enabled them to initiate IND-enabling studies.
Q1: What are the key advantages of using CAR macrophages over traditional anti-fibrotic drugs?
A1: Unlike traditional small molecule drugs that may have broad systemic effects, CAR macrophages offer a highly targeted, cell-based approach. They leverage the natural migratory and phagocytic capabilities of macrophages to infiltrate fibrotic tissue, clear uPAR-expressing cells, and potentially remodel the extracellular matrix, offering a multi-faceted approach to fibrosis resolution with potentially fewer off-target effects.
Q2: What types of projects can benefit most from this service?
A2: This service is ideal for researchers and biopharmaceutical companies engaged in preclinical drug discovery for liver diseases, particularly those focusing on advanced hepatic fibrosis, cirrhosis, or non-alcoholic steatohepatitis (NASH). If your project aims to develop novel, targeted cellular immunotherapies or to validate uPAR as a therapeutic target in fibrosis, our service can provide the critical tools and data you need.
Q3: Can this service be customized for specific patient populations or disease models?
A3: Absolutely. Our anti-uPAR CAR macrophages development service is highly customizable. we can adapt car design, macrophage source, and experimental models to align with your specific research objectives, including exploring allogeneic or autologous approaches and evaluating efficacy in various in vitro and in vivo hepatic fibrosis models. We encourage you to discuss your unique needs with our scientific team for tailored solutions.
To further support your therapeutic development goals, Creative Biolabs offers a range of complementary services that can synergize with our anti-uPAR CAR macrophages development service:
Creative Biolabs is your trusted partner in advancing cutting-edge cell therapies for hepatic fibrosis. Our anti-uPAR CAR macrophages development service for hepatic fibrosis combines scientific excellence with innovative technology to provide you with a powerful tool for therapeutic discovery and development. From initial CAR design to comprehensive functional validation, we are committed to helping you achieve your research milestones. If you are interested in out service, please click the link to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION