Biomarker Analysis Service for ICANS Risk Prediction
Online Inquiry
Introduction Strategies Workflow Required Materials Highlights Publication Customer Reviews FAQs Extended Services
Safeguard Your Pipeline with Precision Diagnostics
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in up to 65% of CAR-T recipients and lacks reliable early predictors. Recent studies highlight the prognostic value of cytokine kinetics (e.g., IL-6, GM-CSF), CSF biomarkers, EEG patterns, and inflammatory gene signatures. Creative Biolabs converts these insights into reliable, accessible biomarker analysis services that support informed decision-making.
Creative Biolabs provides modular and customizable services, including:
-
Multiplex cytokine/chemokine panels for serum and CSF
-
Time-point-based kinetic biomarker quantification (baseline to peak toxicity)
-
Electroencephalogram (EEG) pre/post-infusion signal processing and GTE scoring
-
Machine learning models for personalized ICANS risk prediction
-
Transcriptome-wide cytokine-driven immune cell subset analysis
-
Clinical stratification reports with actionable toxicity guidance
Our services empower teams to de-risk CAR-T programs by identifying high-risk patients, optimizing early interventions, and justifying safety switch inclusion. We provide reproducible, validated, and regulatory-informative biomarker outputs that guide decision-making in both discovery and clinical phases.
Learn How We Can Support You – Schedule a Consultation
Strategies
Our ICANS biomarker framework combines:
-
Peripheral and CSF-based cytokine profiling (IL-1, IL-6, GM-CSF, MCP-1, IFN-γ)
-
Analysis of Inflammatory Risk Signatures Based on CRP, Ferritin, LDH, and D-Dimer Levels
-
Neural injury markers (e.g., neurofilament light, GFAP) in serum/CSF
-
Pre-treatment EEG GTE scoring for electrical vulnerability mapping
-
Transcriptomic and machine learning-based immune clustering
-
Longitudinal kinetic modeling of cytokine release trajectories
This multi-layered strategy integrates dynamic, cellular, and molecular insights to anticipate ICANS onset and severity.
Workflow
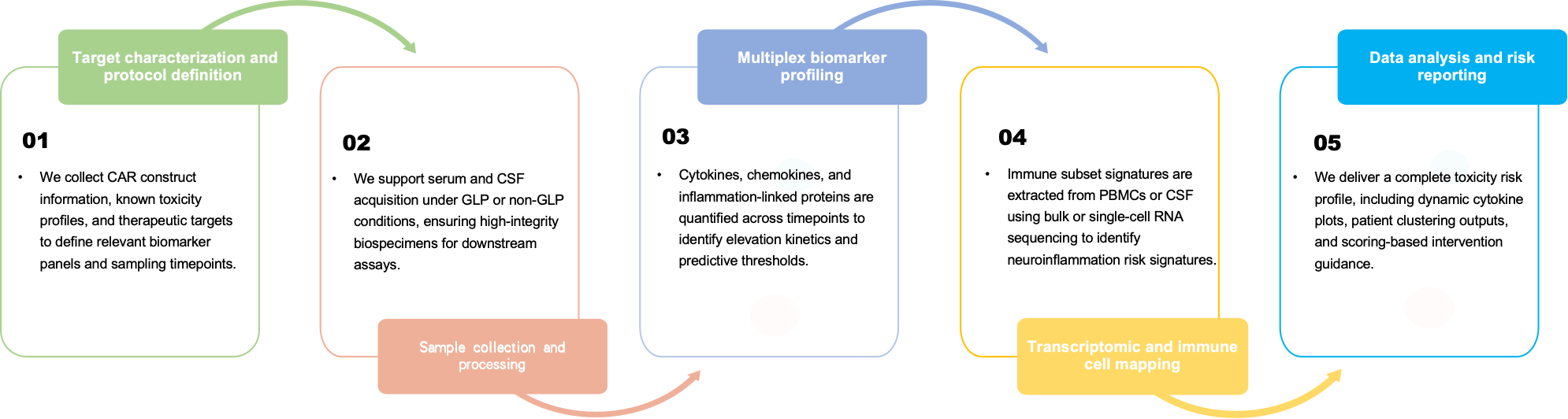
Required Starting Materials
-
CAR target and construct schematic (CD19, BCMA, etc.)
-
Patient cohort metadata (e.g., age, tumor burden, baseline labs)
-
Sample matrix type and collection schedule
-
Preclinical or clinical ICANS observations (if available)
Highlights
Deep immunoneuro signature resolution
We analyze serum and CSF cytokine gradients, integrating them with neural injury markers and immune cell signatures to deliver high-resolution toxicity insights.
Proactive patient stratification
Our risk scoring tools allow early identification of high-risk patients before severe symptoms emerge, enabling tailored monitoring and intervention plans.
Validated across platforms
Assays are compatible with flow cytometry, ELISA, Multiplex bead-based immunoassay, and sequencing workflows, ensuring reproducibility and platform integration flexibility.
Modular and customizable
Each biomarker module can be selected independently or bundled, supporting both exploratory and regulated study requirements.
Discover the Creative Biolabs Difference – Request Your Quote Today
Publication
This diagram presents a systematic framework for organizing known risk factors associated with ICANS. The factors are broadly categorized into host-related, cellular therapy-related, and inflammation-related groups. Notably, certain key elements, such as cytokine release syndrome (CRS), function as bridges across these categories, highlighting their central role in ICANS pathogenesis. Risk factors with the strongest supporting evidence are emphasized in bold, offering a focused foundation for risk assessment, early intervention, and improved clinical management of ICANS.
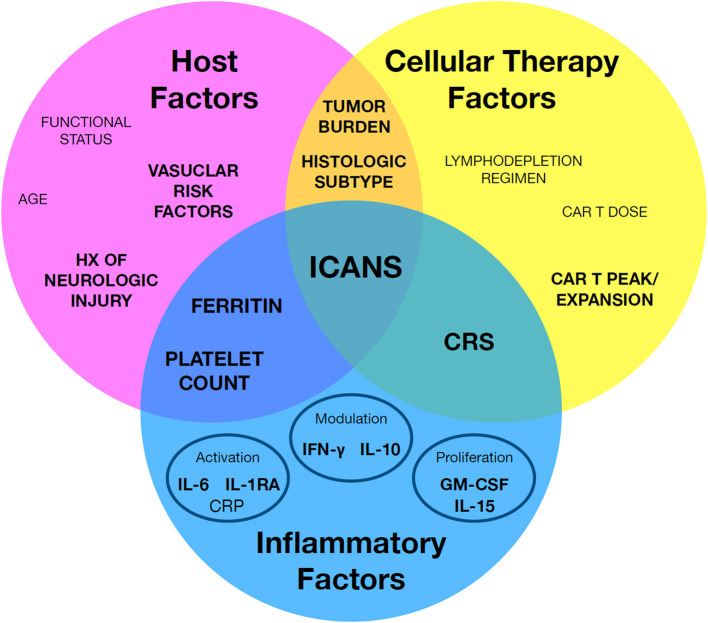 Fig.1 A structured framework for categorizing established risk factors associated with ICANS.1
Fig.1 A structured framework for categorizing established risk factors associated with ICANS.1
Customer Reviews
-
Improved risk prediction
Using Creative Biolabs' biomarker service helped us identify a pre-treatment IL-6 and MCP-1 surge pattern that strongly correlated with ICANS onset in our BCMA CAR-T cohort. Mar 2025, Dr. T*** Chang
-
Enhanced patient selection
Their EEG-GTE analysis allowed us to stratify high-risk subjects before infusion. This changed our monitoring protocol and likely avoided escalation. Jan 2025, Dr. E*** Ruiz
-
Mechanism-informed safety design
The single-cell analysis of cytokine-producing T cell subsets clarified why our dual-target CAR had higher neurotoxicity, leading to a safer revision. Jun 2025, Dr. K*** Lin
FAQs
Can I use this service for both preclinical and clinical studies?
Yes, our biomarker services support both settings and can be scaled to your study design.
Which matrices are accepted for cytokine profiling?
We accept serum, plasma, and CSF. Fresh or cryopreserved formats are suitable depending on the target panel.
Is EEG analysis part of the core service or optional?
It is optional and modular. We can analyze raw EEG data or provide scoring templates for internal use.
How does your machine learning model improve accuracy?
It integrates cytokine kinetics, clinical variables, and neural markers to provide multi-parametric risk classification, outperforming single-marker models.
Extended Services
We provide a quantitative assessment of CAR-T or CAR-NK cell-mediated target cell killing using real-time or endpoint-based methods. It enables functional validation of cytotoxic potency, specificity, and dose-response relationships across different effector-to-target ratios.
T cell activation assay enables precise evaluation of antigen-specific or CAR-induced T cell responses by measuring key activation markers, cytokine secretion, and proliferation. This service supports functional validation of immunotherapies, vaccine candidates, or engineered T cells in both in vitro and in vivo settings.
Creative Biolabs delivers advanced biomarker discovery and ICANS mitigation strategies that empower CAR-T developers to move forward safely and confidently. From discovery to preclinical support, our services are built for precision, flexibility, and speed.
Contact Our Team for More Information and to Discuss Your Project
Reference
-
Butt, Omar H., et al. "A systematic framework for predictive biomarkers in immune effector cell-associated neurotoxicity syndrome." Frontiers in Neurology 14 (2023): 1110647. Distributed under Open Access license CC BY 4.0, without modification.







 Fig.1 A structured framework for categorizing established risk factors associated with ICANS.1
Fig.1 A structured framework for categorizing established risk factors associated with ICANS.1




