Creative Biolabs prepares cancer cancer vaccines through tumor antigen, immune adjuvant, and the vector. Selection of appropriate target antigens, formulations, adjuvants, and delivery pathways are key factors for the success of cancer cancer vaccines. Our experts support cancer vaccine developers and manufacturers by providing advanced characterization programs. We provide comprehensive quality assurance expertise to help you meet and exceed quantity, stability, and purity.
In order to ensure the safety of the cancer vaccine, high standard identification of the test cancer vaccine was carried out from the following aspects:
The attenuation degree of live attenuated cancer vaccine should be considered firstly. Natural virus strains are usually artificially modified to obtain non-pathogenic and immunogenic strains.
It is very extremely crucial to detect allergens in cancer vaccines. By applying current methods, these cancer vaccines do not purify the virus and contain other antigens during cell culture.
Most inactivation methods are chemical inactivators, such as methanol.
With expert stability indicator analytical method development skills, we ensure that any changes in physical and chemical properties, structure, polymerization, biological activity, appearance, impurities, excipient degradation and seal interactions can be detected.
Techniques such as microarray and second-generation sequencing will extract new ideas for evaluating the effectiveness of cancer vaccines.
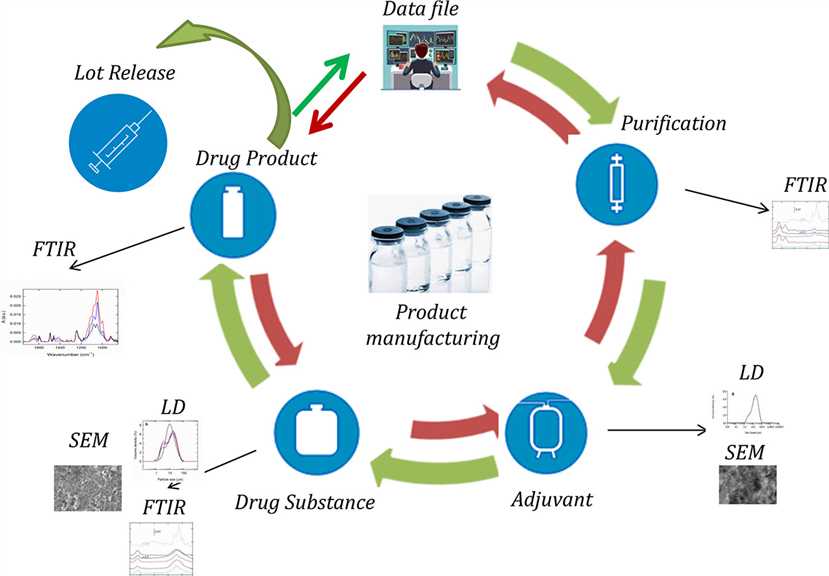 Fig.1 Cancer Vaccine manufacturing.1
Fig.1 Cancer Vaccine manufacturing.1
Creative Biolabs provides a wide range of technologies for you to choose, as shown in the table:
| Chromatographic | Electrophoresis | Immunological |
|
|
|
Table 1: Cancer Vaccine identity technologies.
As stated in the European community directive on infectious biologicals by risk group (2000/54ec), we have specialized laboratory facilities to handle class I and class II biologicals. A variety of fully integrated biosafety testing procedures are used in cancer vaccine identification.
Please feel free to contact us and get more detailed information.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION