To reduce or eliminate the use of animals in titer testing and to ensure the safety of the cancer vaccine, purity testing is carried out for uncertainties in the cancer vaccine production process. Creative Biolabs focuses on exploring and evaluating new tools to develop safe and effective cancer vaccines against pre-existing and emerging viral diseases. To meet FDA standards, the cancer vaccine production process includes extensive process and release testing to ensure consistency in product quality and performance.
Creative Biolabs has the most advanced cancer vaccine purification technology. Each of the following techniques is analyzed by a professional operator, which will help to ensure the purity of the cancer vaccines:
Creative Biolabs has the most advanced cancer vaccine purification technology. Each of the following techniques is analyzed by a professional operator, which will help to ensure the purity of the cancer vaccines:
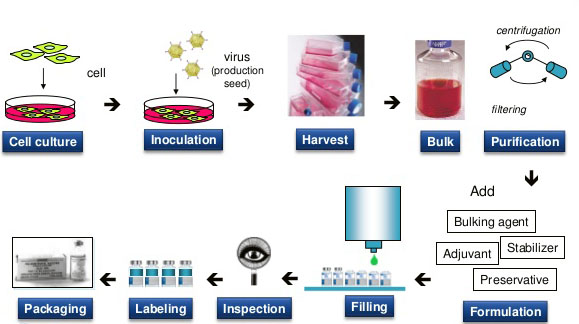 Fig.1 Production of the Cancer Vaccine.
Fig.1 Production of the Cancer Vaccine.
Different cancer vaccines should be separated and purified by different methods, but generally divided into two basic stages: primary and specific separation and purification. The main task of the primary phase of separation is to separate cells and culture media, concentrate the product, and remove most impurities. In the specific purification stage, various high-resolution technologies are selected to isolate the target protein and a small amount of interfering impurities as far as possible, so as to accomplish the required quality standards. Ultra-rapid centrifugation and various chromatographic techniques have become the main methods to achieve this goal.
Creative Biolabs is a leader in cancer vaccine development, focusing on cancer vaccine analysis, development and identification services for many years. Our scientific experts and advanced platforms can provide quality services for you.

The advanced separation and purification technology in the preparation of new cancer vaccines are an effective means for you to choose, which you can acquire to improve the efficacy of cancer vaccines and reduce side effects. Creative Biolabs is a world leader in cancer vaccine analysis and development. With rich experience and advanced platform, we are confident to provide the best development service for Cancer Vaccine purity detection. We can ensure the best results for our customers all over the world.
Please feel free to contact us and get more detailed information.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION