Are you encountering obstacles in accurately validating CAR expression, maintaining consistent product quality, or complying with evolving regulatory standards in your cell therapy development? Creative Biolabs' CAR Expression Test Services deliver end-to-end analytical support to accelerate your CAR-T program. Using state-of-the-art methodologies including high-resolution flow cytometry, quantitative PCR, and proprietary detection reagents, we ensure robust characterization and lot-release readiness. Our tailored solutions provide the critical evidence needed to advance your candidates smoothly through development and toward successful regulatory submission.
The evaluation of CAR expression is a fundamental quality control step in the development of CAR-T cell therapies. High and consistent CAR expression is directly correlated with the efficacy and potency of the therapeutic product. Assessing this expression is critical for optimizing CAR construct design, monitoring transduction efficiency, and ensuring the final product meets the necessary standards for preclinical studies and regulatory submissions.
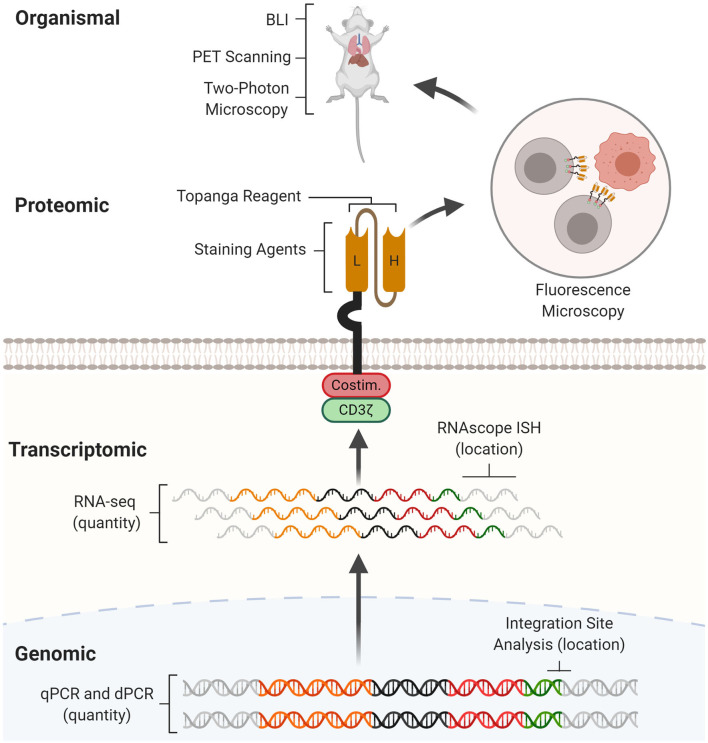 Fig.1 Multiscale assays for CAR expression and function analysis.1,4
Fig.1 Multiscale assays for CAR expression and function analysis.1,4
Creative Biolabs' CAR expression test services employ a comprehensive array of analytical approaches to quantify cell-surface CAR expression, delivering robust data on both the percentage of CAR-positive cells and the intensity of CAR signal. Tailored to the architecture of each CAR and the specific objectives of your study, we provide the most appropriate and rigorously validated detection strategy.
Assessment of CAR expression represents an essential phase in the development of CAR-T cell therapies. Creative Biolabs employs a suite of advanced analytical methods to accurately detect and quantify CAR expression levels, supporting critical quality attribute assessments throughout the therapeutic development process. Available techniques include:
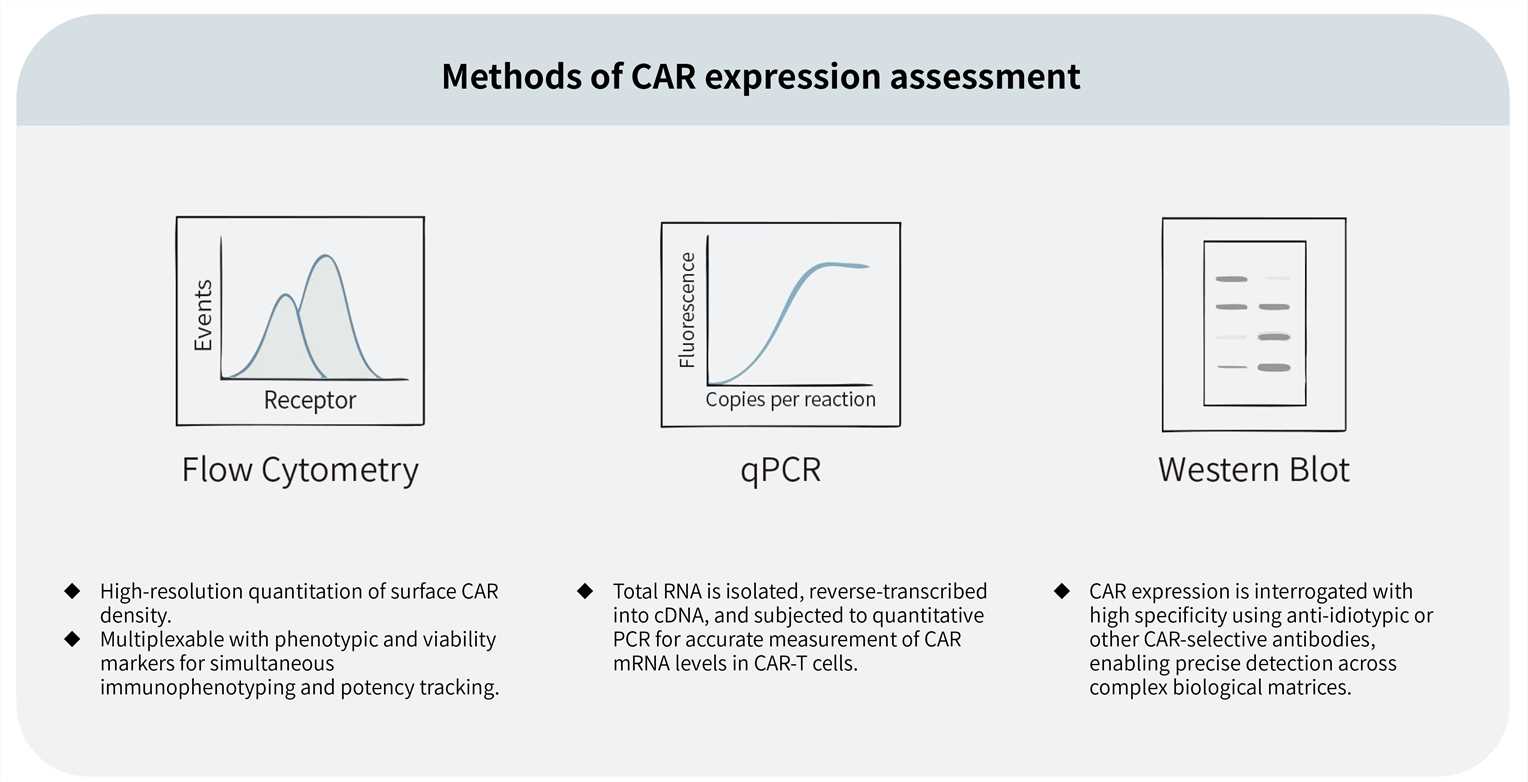
Moreover, we offer bespoke CAR-specific monoclonal antibody development services to detect the expression of the CAR.
Key steps:
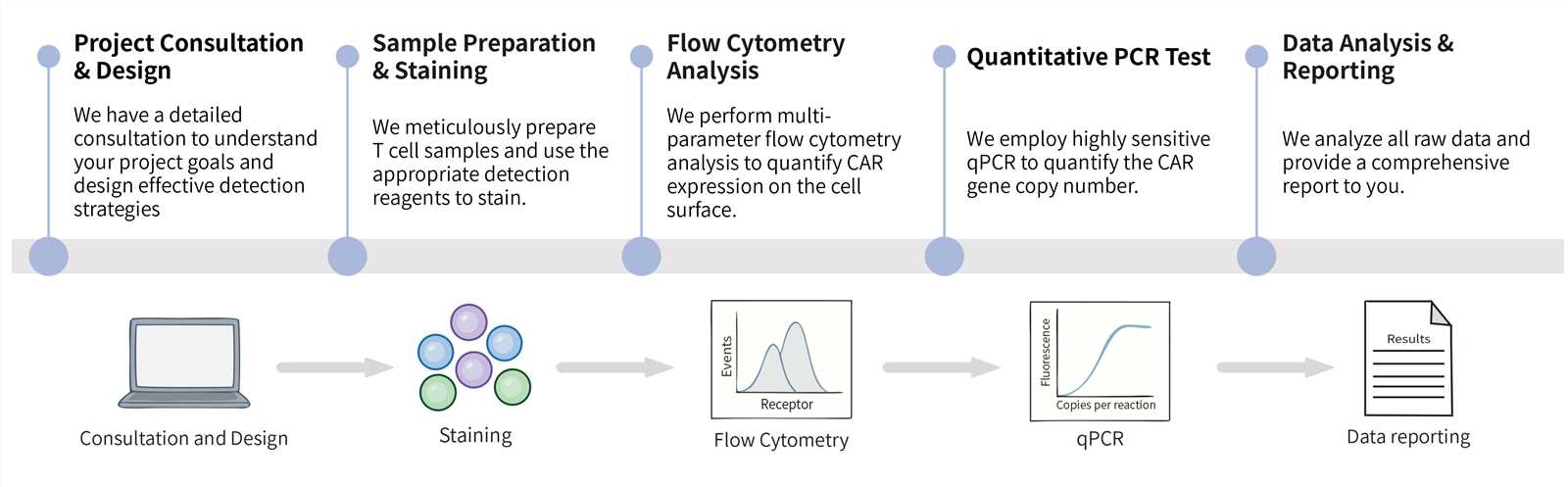
Final Deliverables: Upon project completion, you will receive raw data files, a comprehensive analysis report with supporting graphs and tables, and a final summary report.
The following data exemplify typical experimental outcomes for CAR expression profiling.
Analysis of CAR expression by WB
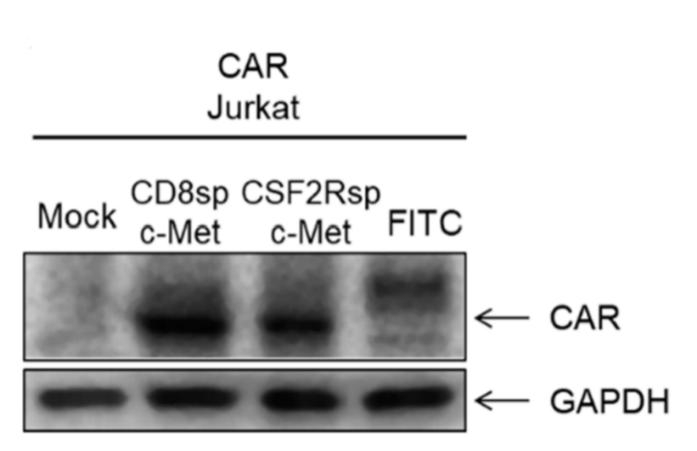 Fig.4 Assessment of CAR expression levels in CAR-modified Jurkat cells.2,4
Fig.4 Assessment of CAR expression levels in CAR-modified Jurkat cells.2,4
Analysis of CAR expression by flow cytometry
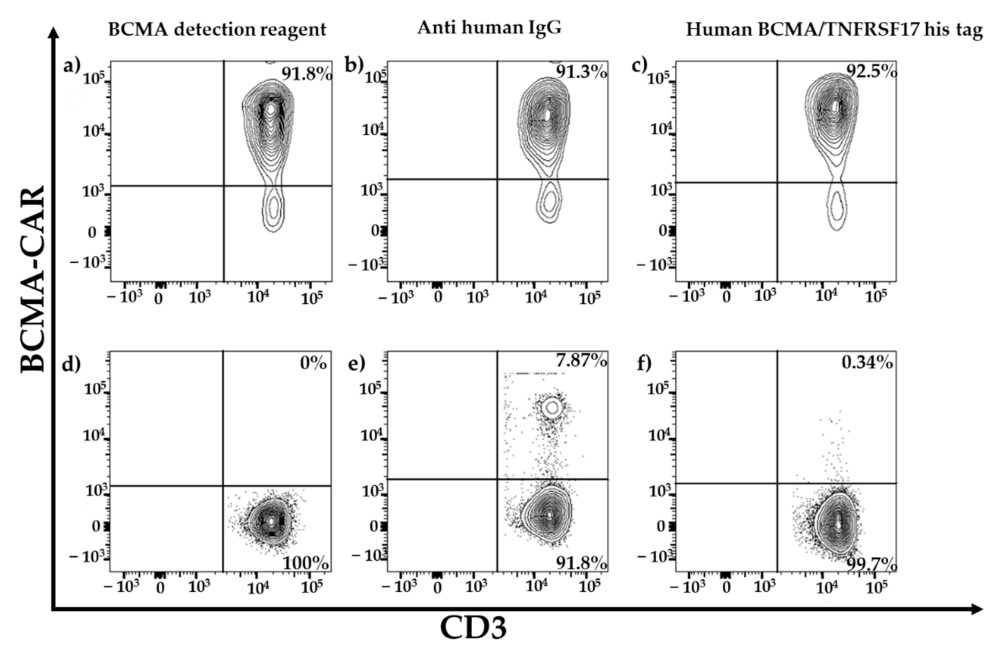 Fig.5 Measurement of CAR expression levels via flow cytometry in BCMA-directed CAR-T cells.3,4
Fig.5 Measurement of CAR expression levels via flow cytometry in BCMA-directed CAR-T cells.3,4
Analysis of CAR expression by flow cytometry and qPCR
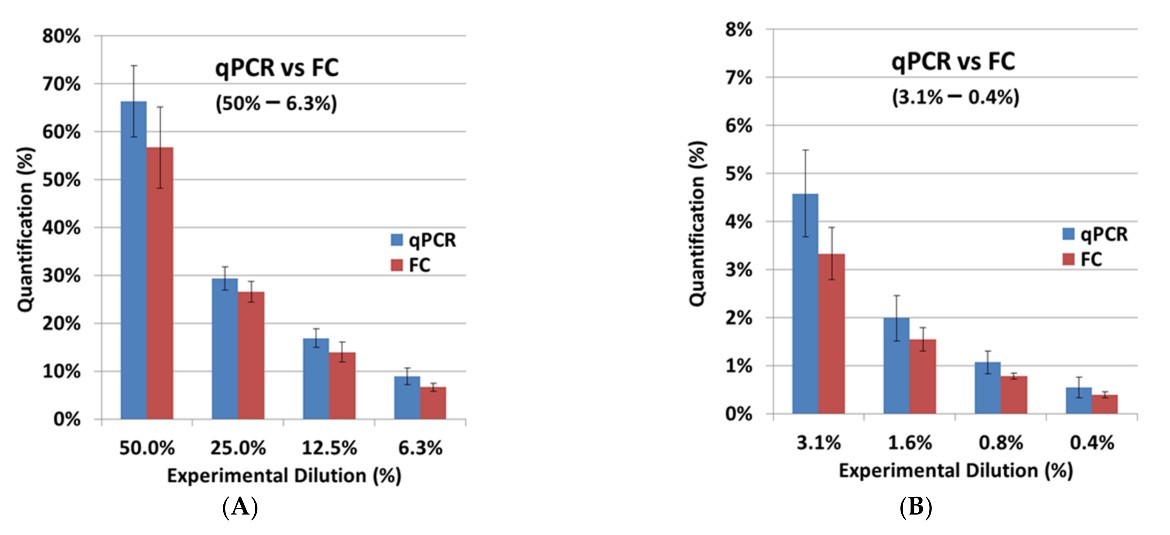 Fig.6 Comparative analysis of CAR detection techniques. 3,4
Fig.6 Comparative analysis of CAR detection techniques. 3,4
What is the best method for testing CAR expression?
There is no universally optimal method for assessing CAR expression, as the selection depends heavily on specific experimental or clinical objectives. Flow cytometry remains the gold standard for quantitative detection of CAR surface protein expression.
Can your service handle a novel CAR with a unique scFv or a new spacer design?
Our services are designed to accommodate high levels of customization. With extensive expertise in protein engineering, we develop tailored detection reagents, including anti-idiotype antibodies and custom universal linker antibodies, to ensure specific and accurate detection of innovative CAR constructs.
Leveraging deep-domain expertise and state-of-the-art analytical platforms, we tailor fit-for-purpose assays to the unique architecture of your CAR construct. Our long-standing experience in assay design and validation consistently delivers precise, reproducible data that robustly underpins every stage of your CAR-T development program.
"The specificity of Creative Biolabs' antigen-based detection reagents is unparalleled. We've seen a significant reduction in the false-positive background staining that plagued our previous assays. It's truly streamlined our quality control workflow. " -J***n S.
"Using Creative Biolabs' CAR Expression Test Services in our research has significantly improved our ability to monitor CAR-T cell persistence at very low concentrations using qPCR. It provides the sensitivity and reliability we need for our long-term in vivo studies." -M***a T.
"The expertise of the Creative Biolabs team was invaluable in developing a custom linker antibody for our unique CAR construct. It saved us months of in-house development time and provided a consistent detection tool across our entire project pipeline." -S***h A.
Our service integrates advanced technologies with deep scientific acumen to deliver reliable, high-resolution, and fully integrated data packages that propel your CAR-T cell therapy programs.
For detailed information or to schedule a project-specific consultation, please contact us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION