As part of our commitment to making CAR-T cell therapies safe and effective, Creative Biolabs is reengineering CAR-T using CMV to create novel cell therapies for solid tumors. We plan to treat solid and hematologic tumors to reduce production costs while improving anti-tumor effects.
Chimeric antigen receptor (CAR) T cells to destroy cancer cells have shown promising roles in treating certain types of cancer, such as leukemia and lymphoma. However, it does not work well in the treatment of solid tumors. Current researchers have discovered a way to overcome this obstacle by using a vaccine to boost the response of CAR T cells and help the immune system produce a new class of T cells that target specific tumor antigens. In studies with mice models, the data have suggested that this vaccine-boosting CAR-T cell approach can not only significantly enhance the killing effect of cell therapy on tumors, but also generate engineered T cell therapy targeting other tumor antigens to further enhance the killing effect on tumors. Moreover, virus-based vaccine-boosting CAR-T cells can restimulate these CAR-T cells and enhance the persistence and antitumor activity.
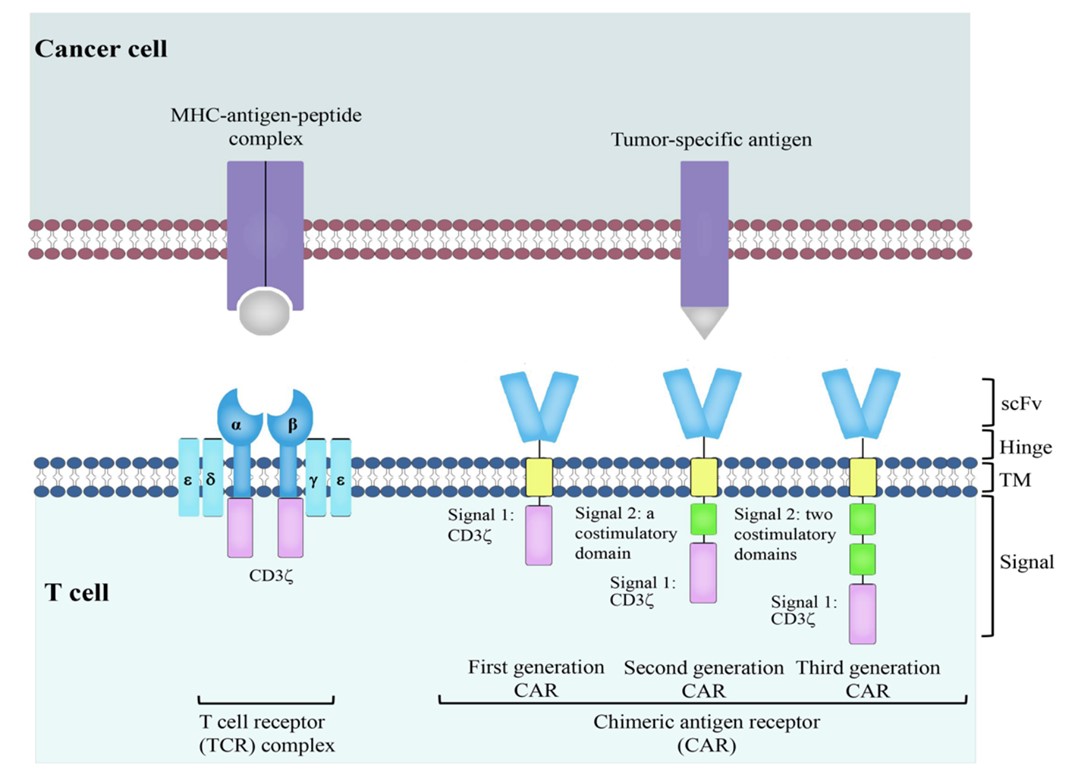 Fig.1 CAR T Cell Design.1
Fig.1 CAR T Cell Design.1
In the past few years, Creative Biolabs has developed a technology platform called CAR-CMVSTs (CAR-modified CMV-specific T Cells) to address the limitations of CAR/TCR t cell therapy in the treatment of solid tumors. Our technology platform is designed to transfer CBL-specific endogenous cytomegalovirus (CMV) specific T cell receptors into existing CAR T cells to provide more durable antitumor activity.
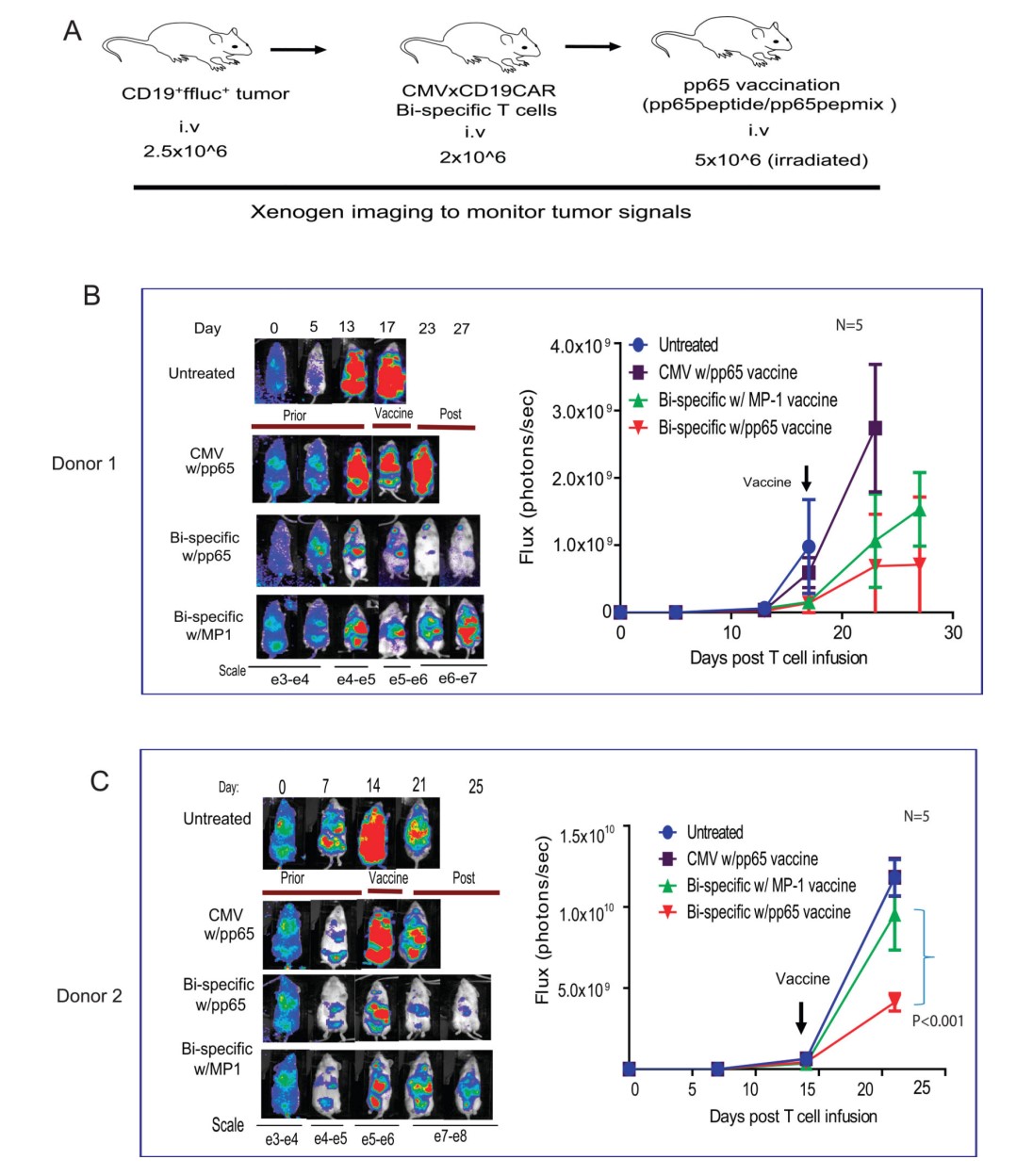 Fig.2 Anti-Tumor Activity of Adoptively Transferred Bi-Specific T Cells is Enhanced by CMVpp65 Vaccination.2
Fig.2 Anti-Tumor Activity of Adoptively Transferred Bi-Specific T Cells is Enhanced by CMVpp65 Vaccination.2
Nowadays, we have redirected CMV-specific CAR-T cells to recognize and lysate tumor cells via a range of tumor-associated targets, including but not limited to CD19 and HER2. Our unique CMV-specific CAR-T cells can maintain their ability to proliferate in response to CMV antigen stimulation and improve antitumor efficacy in B-cell malignancy models.
Creative Biolabs employs key proprietary technologies that work together to enable us to develop the most effective and safe CAR-CMVST cell therapies. Furthermore, we have generated best-in-class CMV peptide-HLA complex delivery solutions to address specific unmet needs in the field of cell therapy. We have a proprietary set of CAR engineering technologies, dual CAR and enhanced CAR, that can be combined with our CMV-peptide technology platform to further enhance the therapeutic efficacy of our CAR-T product candidates.
Creative Biolabs is developing advanced CAR-CMVST cell therapies to deliver powerful, targeted, effective novel CMV-specific CAR-T cell therapies to harness the body's immune system and cure cancer. We have 2000 sq meters of GMP facilities in the USA. Our scientists have published more than 100 peer-reviewed papers in leading international journals. If you are interested in our services, please contact us for more details. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION