We design pan-ErbB CAR-T cells for comprehensive pathway targeting. Our services include construct engineering and preclinical validation, addressing redundancy in ErbB-driven cancers and improving therapeutic efficacy.
CAR-T immunotherapy is a robust treatment strategy for hematologic malignancies with dramatic anti-tumor efficacy. However, the expansion and trafficking rate of CAR-T cells are often insufficient to produce long-term remission, and serious CRS toxicities may occur after administration. The imaging platform development for CAR-T cell expansion and trafficking visualization will provide a promising approach to monitor inadequate response and act as an early warning for CAR-T toxicity, allowing CAR-T therapy to be tailored accordingly to maximize therapeutic efficiency.
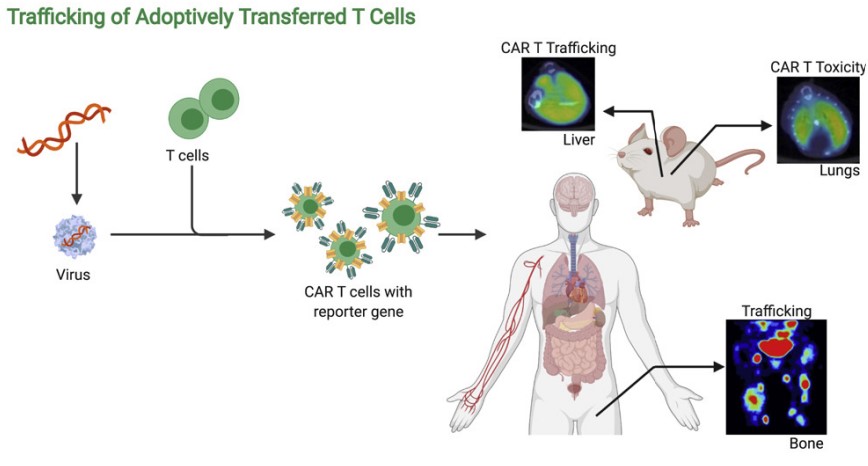 Fig.1 The schematic of in vivo CAR-T imaging.1
Fig.1 The schematic of in vivo CAR-T imaging.1
To this end, Creative Biolabs is committed to providing comprehensive CAR-T imaging services for global customers to facilitate the tracking of CAR-T cells in vivo. Our services cover non-specific strategies to visualize activated T cells and robust reporter systems to specifically monitor engineered T cells. These approaches are expected to provide valuable information and guidance to improve CAR-T therapy.
Our CAR-T imaging services cover a variety of non-specific approaches to detect activated T cells, and many robust reporter systems to specifically monitor engineered T cells, including but not limited to the following sections:
Non-specific CAR-T Imaging Service of Activated T cells:
In vivo activated T cell imaging utilizing surface activation markers:
CD25, OX40, PD-1, CTLA-4, Granzyme B, IFN-γ;
In vivo activated T cell imaging utilizing metabolic pathways kinase:
Deoxycytidine kinase (dCK), Deoxyguanosine kinase (dGK);
In vivo activated T cell imaging utilizing T cell-specific surface markers:
TCR, CD3, CD4, CD8;
Specific CAR-T Imaging Service using Reporter Genes:
Herpes simplex virus 1 thymidine kinase (HSV-tk);
Sodium iodide symporter (NIS);
Somatostatin receptor type 2 (SSTr2);
Human deoxycytidine kinase (hdCK);
Our comprehensive service workflow is meticulously designed to move your universal CAR candidate from concept to preclinical validation efficiently and reliably.
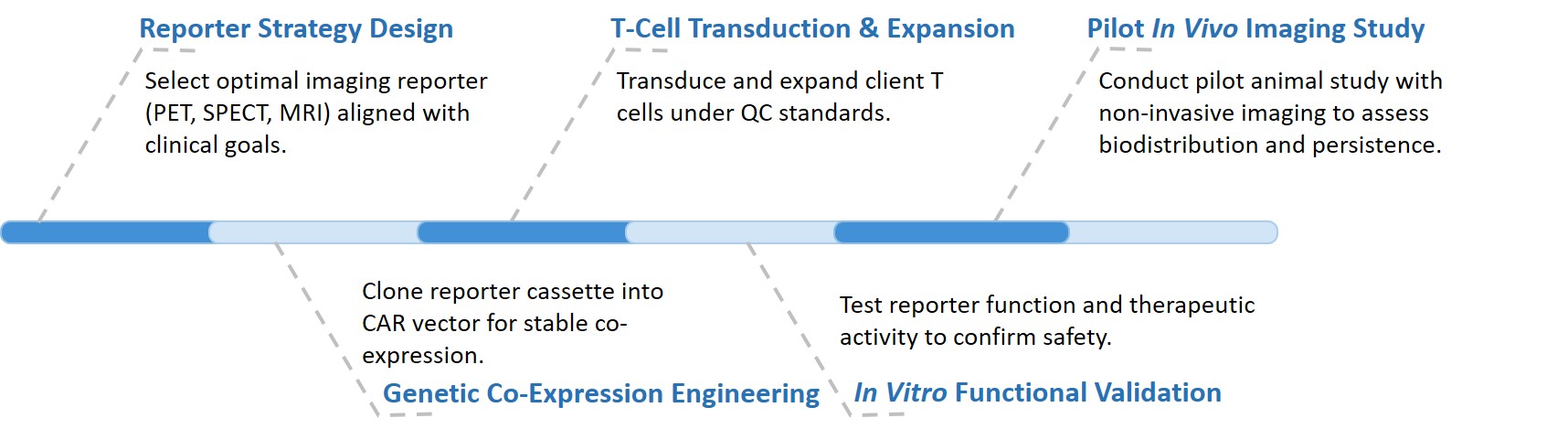
Final deliverables include a biodistribution report, a QC-certified CAR-T cell bank, and validation confirming stable, safe reporter integration without functional loss.
Contact our team to fully go over your one-of-a-kind requirements.
We design pan-ErbB CAR-T cells for comprehensive pathway targeting. Our services include construct engineering and preclinical validation, addressing redundancy in ErbB-driven cancers and improving therapeutic efficacy.
Creative Biolabs engineers universal CARs combining NKG2D and TIM. Our services address adaptability, providing broad tumor coverage and enhanced flexibility for diverse applications.
Our team constructs peptide-centric CARs, expanding targeting beyond conventional antigens. Services address antigen diversity, enabling new recognition strategies and supporting broader therapeutic development.
In vivo CAR-T imaging with nanoparticle labeling
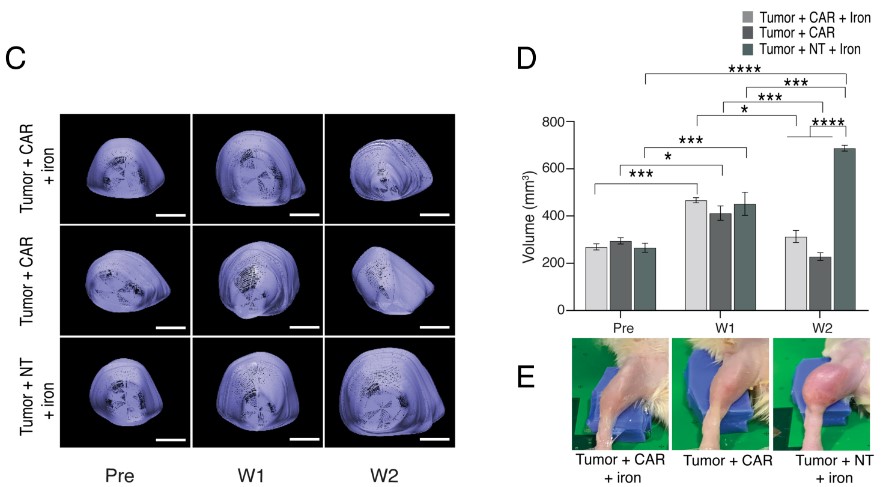 Fig.2 Photoacoustic images of CAR-T cells visualization labeled with ferumoxytol.2
Fig.2 Photoacoustic images of CAR-T cells visualization labeled with ferumoxytol.2
Q1: What is the significance of CAR-T imaging development?
A1: As CAR-T therapy continues to become an important means of cancer treatment, there will be an urgent need to develop several appropriate imaging platforms to allow non-invasive imaging and monitoring of the expansion and trafficking rate of CAR-T cells in vivo. CAR-T cell dynamics visualization is of great significance in studying the interaction between T cells and cancer cells, as well as several normal tissues.
Q2: What criteria should an ideal CAR-T imaging platform meet?
A2: Such a CAR-T imaging platform meets the following criteria:
 Fig.3 Work with Creative Biolabs.
Fig.3 Work with Creative Biolabs.
Creative Biolabs provides comprehensive in vivo CAR-T imaging services that allow for full customization and design from pilot studies to special targeted projects. If you are interested in more information about our in vivo CAR-T imaging service, please don't hesitate to contact us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION