Chimeric Antigen Receptor (CAR) T-cell therapy has heralded a new era in oncology, delivering unprecedented clinical responses for patients grappling with aggressive hematological malignancies, including relapsed or refractory B-cell acute lymphoblastic leukemia, various forms of B-cell lymphoma, and multiple myeloma. However, the powerful immune response utilized by CAR-T cells, although beneficial in treatment, may also trigger a series of serious adverse events. Among these, neurotoxicity stands as a critical challenge, demanding sophisticated understanding and targeted management strategies to ensure patient safety and maximize therapeutic efficacy. At Creative Biolabs, our two decades of specialized expertise in biological research position us at the forefront of addressing this complex frontier.
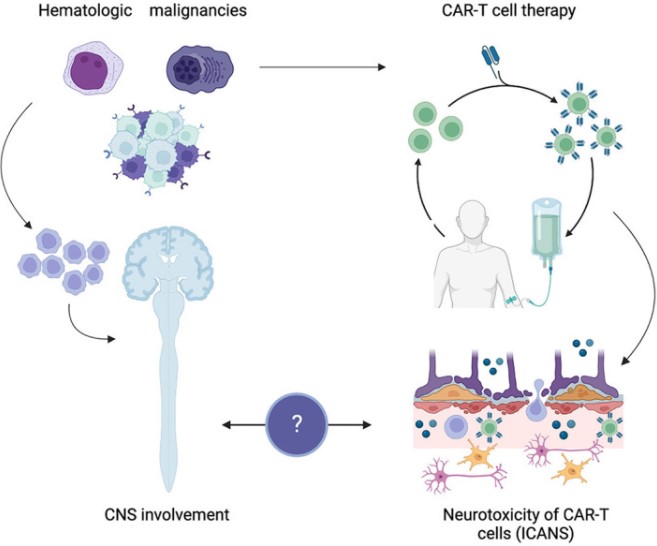 Fig.1 Neurotoxicity in CAR-t cell-associated CNS hematologic diseases.1
Fig.1 Neurotoxicity in CAR-t cell-associated CNS hematologic diseases.1
The successful navigation of CAR-T associated neurotoxicity hinges on a profound understanding of its underlying mechanisms. This requires rigorous preclinical investigation, for which CAR-T model studies are indispensable. These sophisticated models allow researchers to dissect the complex interplay between activated CAR-T cells, systemic inflammation, and the delicate neurological environment.
The primary neurotoxic syndromes observed post-CAR-T infusion include: Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), and Tumor Inflammation-Associated Neurotoxicity (TIAN). Through meticulous in vitro and in vivo model systems, Creative Biolabs' services facilitate the identification of crucial predictive biomarkers and risk factors for these neurotoxic events. For instance, preclinical insights have highlighted that high tumor burden and early-onset or high-grade CRS can serve as significant risk factors for subsequent ICANS development, guiding proactive clinical management.
Creative Biolabs offers a comprehensive suite of CAR-T model related neurodamage study services, meticulously designed to accelerate the development of safer and more effective cellular immunotherapies. Our offerings are grounded in scientific rigor and leverage our extensive experience:
A critical component of understanding CAR-T related neurotoxicity involves thoroughly characterizing the phenotypic changes induced in preclinical models. Creative Biolabs provides comprehensive assessments of neurological dysfunction, including detailed behavioral evaluations (e.g., locomotor activity, cognitive function tests), electrophysiological recordings (e.g., EEG for seizure activity), and advanced imaging techniques (e.g., MRI for brain edema or inflammation). These assessments allow for precise quantification of neurodamage severity, progression, and response to therapeutic interventions, offering crucial translational insights into clinical manifestations of ICANS and TIAN.
Delving into the molecular underpinnings of CAR-T neurotoxicity, our gene discovery service utilizes cutting-edge genomics and transcriptomics approaches. We identify key genes and pathways that are dysregulated during neurotoxic events, providing a deeper understanding of disease mechanisms. This service is invaluable for identifying novel therapeutic targets and biomarkers specific to CAR-T induced neurodamage, facilitating the development of highly targeted and effective mitigation strategies.
With over 20 years of dedicated experience in biological research, Creative Biolabs stands as a trusted partner in the complex field of CAR-T cell therapy development. Our distinct advantages include:
Q1: What types of CAR-T neurotoxicity models does Creative Biolabs offer?
A1: Creative Biolabs utilizes a range of advanced in vitro and in vivo models, including specialized mouse models engineered to mimic CAR-T induced neuroinflammation and dedicated CNS tumor models for investigating tumor inflammation-associated neurotoxicity (TIAN).
Q2: How does Creative Biolabs ensure the scientific rigor of its studies?
A2: Creative Biolabs adheres to the highest scientific standards, employing robust experimental design, stringent quality control measures, and rigorous data analysis. Our experienced team ensures that all studies are conducted with precision and accuracy, yielding reliable and reproducible results.
Q3: Can Creative Biolabs assist with biomarker discovery for ICANS?
A3: Yes, Creative Biolabs offers comprehensive services for the discovery and validation of predictive biomarkers for ICANS. Leveraging advanced omics technologies and bioinformatics, we help identify molecular signatures that can predict patient risk and guide early intervention strategies.
At Creative Biolabs, we are dedicated to advancing the safety and efficacy of CAR-T cell therapies through cutting-edge scientific research and unparalleled expertise in neurodamage studies. Partner with us to navigate the complexities of CAR-T associated toxicities and accelerate your journey towards transformative cancer treatments.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION