CAR-T therapy has shown impressive results in treating hematological malignancies. However, this groundbreaking immunotherapy is associated with significant adverse events, notably neurotoxicity. CAR-T-cell-associated neurotoxicity (CAR-T-NT) presents a spectrum of neurological complications, ranging from mild encephalopathy to life-threatening cerebral edema and seizures. The precise mechanisms underlying CAR-T-cell-associated neurotoxicity are still being investigated, hindering the development of effective preventative and therapeutic strategies.
The ability to predict, diagnose, and manage CAR-T-NT is crucial for maximizing the benefits of this therapy while minimizing its risks. The recent developments in genomics have identified specific genetic factors that may predispose patients to CAR-T-NT or influence its severity. Understanding the genetic basis of CAR-T-NT holds the key to personalized risk assessment, early detection, and the development of targeted interventions.
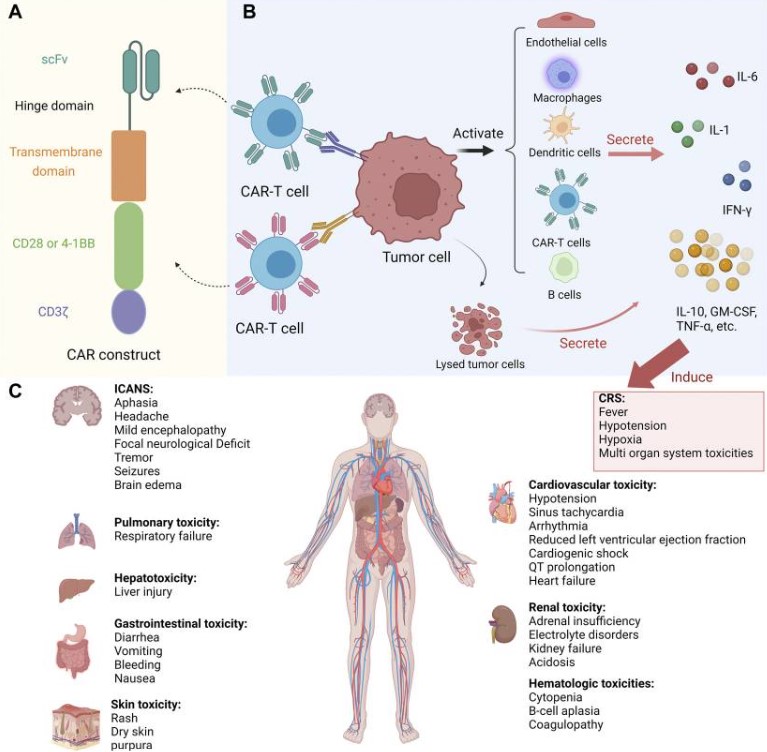 Fig.1 CAR-T therapy toxicities.1
Fig.1 CAR-T therapy toxicities.1
Creative Biolabs offers a cutting-edge Neurodamage Specific Gene Discovery Service designed to address the critical need for improved CAR-T-NT management. This service leverages our extensive expertise in genomics, bioinformatics, and neurobiology to identify and validate genetic biomarkers associated with the development and severity of CAR-T-NT.
Creative Biolabs' Neurodamage Specific Gene Discovery Service encompasses a comprehensive suite of methodologies:
Our team is currently undertaking extensive genome-wide association studies (GWAS) to identify common genetic variants that may influence a patient's susceptibility to CAR-T-related neurotoxicity (CAR-T-NT). This involves analyzing DNA from cohorts of CAR-T-treated patients with and without neurotoxicity, using advanced statistical methods to pinpoint significant genetic associations.
We perform whole-exome sequencing to identify rare or novel coding variants that may contribute to CAR-T-NT. This method mainly focuses on the protein-coding regions of the genome, where the majority of disease-causing mutations reside.
Creative Biolabs utilizes RNA-Seq to analyze gene expression profiles in relevant tissues (e.g., cerebrospinal fluid, brain biopsies, peripheral blood mononuclear cells) from CAR-T-treated patients. This provides insights into the transcriptional changes that occur during neurotoxicity and helps identify genes that are dysregulated.
Our team of expert bioinformaticians employs state-of-the-art algorithms and databases to analyze the vast amounts of genomic and transcriptomic data generated.
Creative Biolabs goes beyond mere association studies. We perform functional validation experiments to confirm the role of candidate genes in CAR-T-NT.
We use rigorous statistical methods and validation cohorts to identify robust and reliable genetic biomarkers.
We offer customizable service packages tailored to specific project requirements, including sample processing, data analysis, and reporting.
Q1: What sample types are suitable for this service?
A1: We can work with a variety of sample types, including DNA from blood or tissue, RNA from cerebrospinal fluid (CSF), brain biopsies, and peripheral blood mononuclear cells (PBMCs).
Q2: What kind of data output is provided?
A2: We provide comprehensive reports with detailed data analysis, including variant lists, gene expression profiles, pathway analysis results, and functional validation data, all delivered in industry-standard formats.
Creative Biolabs is committed to advancing the understanding and management of CAR-T-NT through innovative gene discovery solutions. Please feel free to reach out to us to discuss the specifics of your project. We would be happy to explain how our expertise can help accelerate your research and contribute to improved patient outcomes.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION