γδ T cells expanded and transduced from blood, which requires the ability of favorable migration toward tumor cells for adoptive cell therapy. Creative Biolabs provides cell migration assay to help our clients determine whether the presence of the CAR is critical for migration.
Adoptively transferred or endogenous γδ T cells need to infiltrate into the tumor to exert their antitumor activity. γδ T cells provide a migratory advantage over αβ T cells. Major tumor-infiltrating γδ T cell subsets are human Vδ1 and Vγ9Vδ2 T cells. Both of them have the cytotoxic capability and can have anti-cancer activity and display distinct chemokine receptors that bestow these cells the property to migrate to the tumor site. Most γδ T cell immunotherapy development currently focuses on Vδ2 cells. Vγ9δ2 T cells are a major subset in the peripheral blood. Most of the Vγ9δ2 γδ T cells have canonical TCRs responding to prenyl pyrophosphates that are elevated in cancer cells and are recruited into the tumor via chemokine receptors.
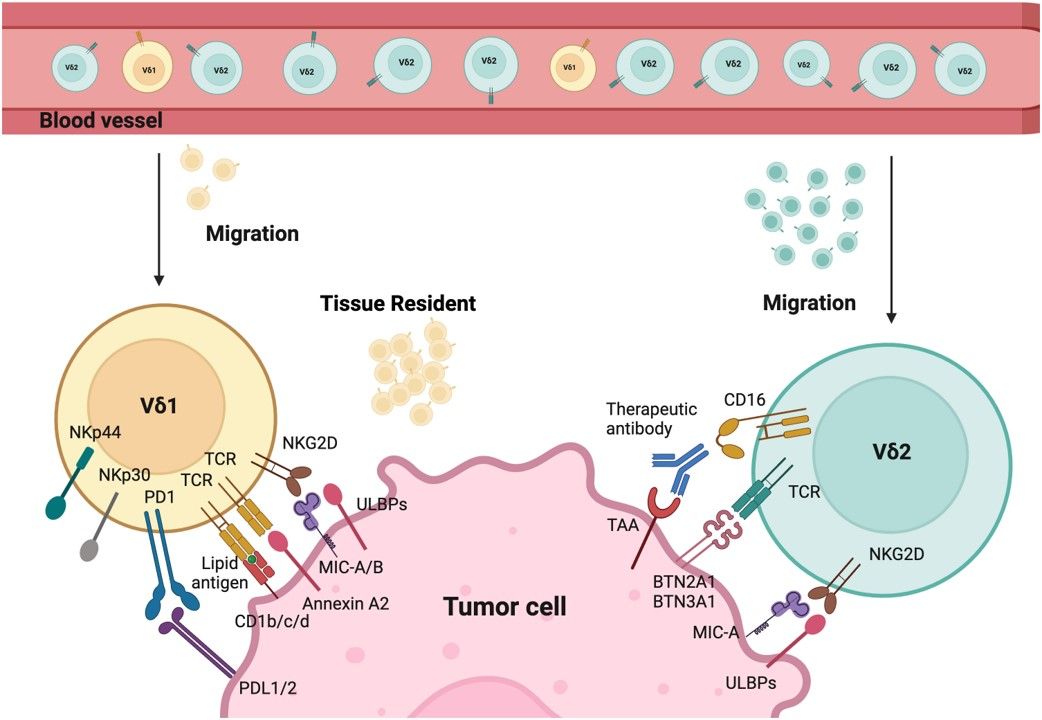 Fig.1 Tumor targeting mechanisms of Vδ1 and Vδ2.1
Fig.1 Tumor targeting mechanisms of Vδ1 and Vδ2.1
Cell migration assay can certain whether the inherent migration tropism of γδ T cells is affected by prolonged culture or transduction with CAR gene. Popular cell migration assays typically are carried out using transwell migration assay. Cells are cultured inside the chamber and enabled to migrate through the pores, to the other side of the membrane. The migratory potential of γδ T cells populations is determined by a chemotaxis assay using 24/96-well culture plates carrying polycarbonate membrane-coated transwell permeable inserts. Cells are seeded in the upper wells and lower wells contain tumor cell stimulus or not. Counting beads are added to count the number of migrated cells. T cell motility in the absence of any cells in the lower chamber is used as background migration. The background values are subtracted from migration in the presence of a tumor cell stimulus to identify stimulus-specific migration.
As a leader who has been devoted to CAR-T therapy development over a decade, Creative Biolabs is confident in providing services with the best quality to accelerate the success of your program. We will work closely with you step by step to ensure the most reliable and accurate outcomes. Please feel free to contact us for more detailed information.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION