Target Background
CD16 is a cluster of differentiation molecule expressed on the surface of natural killer (NK) cells, monocytes, macrophages and neutrophil polymorphonuclear leukocytes. It is identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b) and involved in antibody-dependent cellular cytotoxicity (ADCC). Binding of CD16 to the Fc domain of IgG antibodies result in cross-linking of CD16 on the surface of effector cells such as NK cells. This cross-linking induces increased intracellular Ca2+ levels and a cascade of signal transduction and eventually activates the effector cells.
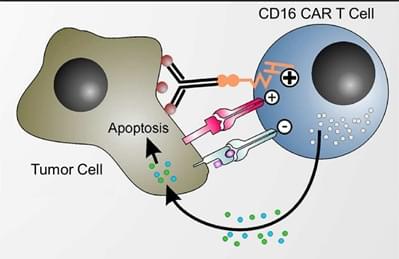 Fig.1 Antibody-dependent cellular cytotoxicity in therapeutic antibody treatment.1
Fig.1 Antibody-dependent cellular cytotoxicity in therapeutic antibody treatment.1
CD16 CAR-T Cell Therapy
In a preclinical study, a CAR construct consisting of a high-affinity CD16 variant, hinge and transmembrane domains, and signal domains from CD3ζ and 4-1BB (TNFRSF9) is transduced into T cells. These CAR-T cells, which are administrated in combination with therapeutic monoclonal antibodies (mAbs), specifically kill the tumor cells at a low effector-target ratio. Similar results are obtained with many other mAbs such as anti-CD20 antibody, anti-HER2 antibody and anti-GD2 antibody. Therefore, the CD16 CAR has the potential to be a universal chimeric receptor to enhance the efficacy of therapeutic antibodies for cancer treatment. In order to validate this discovery, patients with B cell malignancies are given autologous CD16 CAR-T cells in combination with anti-CD20 mAb to assess the feasibility, safety and efficacy of the therapy.
Animal Models for in vivo Study of CD16 CAR-T Cell Therapy
The animal models used for CD16 CAR-T cell therapy research are developed by the target of co-administered monoclonal antibody. Different kinds of cancer models have been established for the in vivo assay of CD16 CAR-T cells in our facility. Our scientists use multiple kinds of cancer cell lines or human primary cancer cells to develop xenograft cancer models in immunodeficiency mice. We also provide rats and non-human primates (NHPs) models to meet your special requirement.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform forCD16 CAR-T cell therapy research.
Efficacy Test
Tumor remission monitored by tumor cell analysis or bioluminescence imaging and survival curve tracking.
Viability and Bio-distribution Studies
Durability, bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, The route of administration, Dose regimen/response/onset)
Clinical observation (body weight, feed consumption, ophthalmologic and clinical pathology)
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Complete necropsy, organ weight
Histopathology
Tumorigenicity study
Quality Control for In Vivo Assays
All our experiments are performed by well-trained and experienced technicians in a Quality Control for In Vivo Assays Service.
Creative Biolabs is ready to provide assistance for your CD16 CAR-T preclinical in vivo assay. Our exceptional team of scientists design and perform CAR-T assays ranging from development of animal models to the evaluation of efficacy, safety, viability and bio-distribution of CD16 CAR-T cells. We also offer a broad range of animal models including rats and non-human primates (NHPs).
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION