Are you currently facing bottlenecks in producing high-quality viral vectors for your gene therapy research or clinical trials? Our clinical-grade viral transgene vector manufacturing service helps you accelerate your gene therapy development through reliable, scalable, and highest industry practice-compliant viral vector production.
By using therapeutic genetic material to treat specific cells, gene therapy offers great promise in addressing various diseases. Maintaining both the accuracy and safety of this genetic transfer depends on viral delivery methods still being essential. Clinical trials confirming the efficacy of gene-based medicines highlight how quickly progress in this field is occurring. Thus, maintaining strict safety standards and meeting growing therapeutic needs depend on the development of dependable, scalable manufacturing processes for clinical-grade viral carriers.
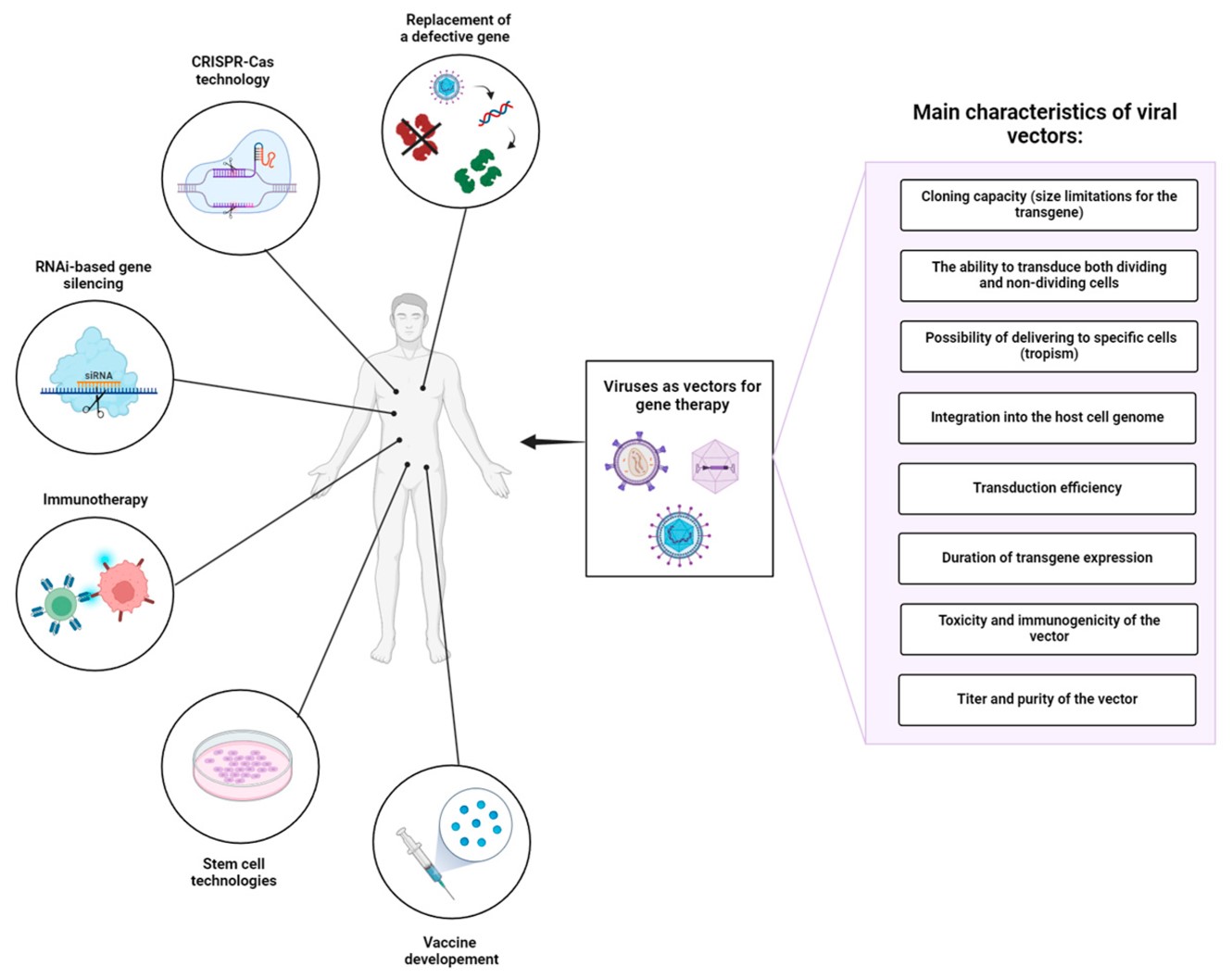 Fig.1 Important factors that influence the selection of viral vector platforms and their application in gene therapy.1
Fig.1 Important factors that influence the selection of viral vector platforms and their application in gene therapy.1
Creative Biolabs offers an efficient and dependable clinical-stage viral vector manufacturing service, customized to meet distinct project specifications. Our integrated approach resolves technical challenges associated with clinical-grade vector manufacturing, ensuring compliance with the highest industry practice. Recognizing that each project presents unique demands for vector architecture, scalability, and quality benchmarks, we collaborate closely with clients to deliver tailored strategies. These encompass every phase, from preliminary design consultations through to final product formulation and packaging. By serving as collaborative partners, we facilitate the seamless progression of initiatives, optimizing efficiency at each stage. Our specialized proficiency spans multiple vector platforms, allowing broad support for varied gene therapy development efforts.
Our specialists work closely with clients to define research objectives, refine transgene delivery constructs, and identify optimal viral delivery systems tailored to specific experimental needs.
High-purity master and working cell banks are generated under stringent industry-standard conditions, providing standardized, traceable starting materials for scalable vector manufacturing.
Advanced bioreactor systems and validated purification methods enable high-yield vector synthesis, coupled with multi-stage filtration to eliminate residual impurities and ensure product integrity.
All vector lots undergo systematic evaluation of genomic titer, biological potency, sterility, and absence of microbial contaminants, meeting global pharmacopeia requirements for therapeutic applications.
Quality-certified vectors are sterile-packaged in client-specified formats using temperature-stable materials, with customized labeling for secure global distribution.
Q1: Which viral vectors are available for clinical-grade production at Creative Biolabs?
A1: Creative Biolabs' capabilities encompass the production of multiple clinically compliant viral vectors, such as adeno-associated virus (AAV), lentivirus, adenovirus, and additional variants. Consult our team to evaluate your project's vector specifications and alignment with our services.
Q2: How is quality assurance ensured during production?
A2: Manufacturing strictly follows the highest industry practice, with analytical testing integrated across all phases--from raw material qualification to batch release. These protocols guarantee vector safety, efficacy, and consistency. Our team is available to clarify specific quality assurance methodologies.
Q3: Does Creative Biolabs support bespoke vector designs and scalable production?
A3: Recognizing the distinct demands of gene therapy development, we provide adaptable solutions for custom vector engineering, transgene integration, and scalable manufacturing. Please contact us to review your objectives and identify optimized workflows.
Q4: What are the expected timelines for clinical-grade vector production?
A4: Project duration is influenced by vector complexity, batch size, and analytical requirements. While prioritizing quality, we strive to expedite processes where feasible. Submit your parameters to receive a project-specific schedule.
Creative Biolabs is a reliable associate with a wealth of experience in the provision of superior biological services. We are dedicated to delivering scalable and dependable solutions that expedite the development of your gene therapy. With our dedication to regulatory compliance, client satisfaction, and quality, we are the optimal choice for your clinical-grade viral vector manufacturing requirements. Please do not hesitate to contact us if you are interested in our clinical-grade viral transgene vector manufacturing service.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION