Creative Biolabs provides a cord blood CD34+ dendritic cell (DC) production service that enables robust and scalable generation of high-quality dendritic cells from cord blood–derived progenitors, supporting reliable modeling of human immune signaling and antigen presentation and facilitating the study of immune pathways, immunomodulatory agent screening, and vaccine development.
Cord blood-derived CD34+ hematopoietic progenitors offer a potent source for generating dendritic cells ex vivo. High-performance isolation and cytokine-mediated protocols yield functional DCs suited for immunological studies, with demonstrated scalability and phenotype stability. These advances underpin our production service, ensuring both quality and reproducibility.
Creative Biolabs' cord blood CD34+ hematopoietic stem/progenitor cell (HSPC)-derived DC production service provides a robust and scalable solution for obtaining large quantities of highly functional dendritic cells. We address the critical need for consistent, high-quality DCs in diverse research and therapeutic applications, including cancer immunotherapy, infectious disease studies, and immunogenicity testing. Our service delivers phenotypically mature and functionally potent DCs tailored to your specific project requirements, ensuring reliable performance in downstream applications. This translates to accelerated research timelines and enhanced confidence in your experimental outcomes.
| Cell Sourcing and Processing | DC Differentiation and Expansion |
|---|---|
|
|
| Phenotypic and Functional Analysis | |
MHC molecules Integrins and adhesion molecules Lineage markers |
|
Summary: Focused on identifying the molecular markers of naive CD34−/CD34+ hematopoietic stem/progenitor cell populations, this research analyzed samples from cord blood and bone marrow. Cord blood had more Lin−CD34−CD38 Low/− cells with lower aldehyde dehydrogenase, reduced clonogenicity, but potent megakaryocyte/erythrocyte differentiation. Proteomics revealed 85.6% similarity and 14.4% differential proteins; subsets exhibit distinct traits, with potential novel markers.
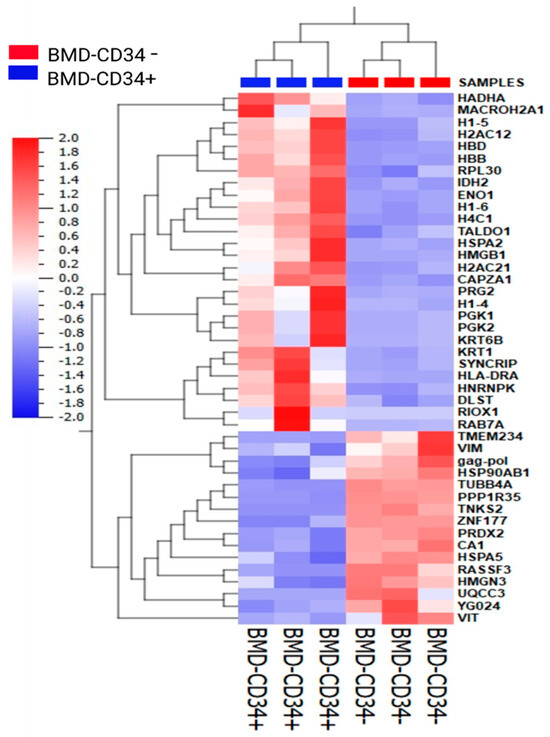 Fig.1 Hierarchical cluster analysis for proteins associated with CD34− and CD34+ populations.1
Fig.1 Hierarchical cluster analysis for proteins associated with CD34− and CD34+ populations.1
Q1: Why choose cord blood CD34+ cells over other sources for DC generation?
A1: Cord blood CD34+ cells offer several advantages, including high proliferative potential, ethical accessibility, and reduced immunogenicity compared to adult sources. This makes them ideal for scalable and consistent DC production for various therapeutic and research applications.
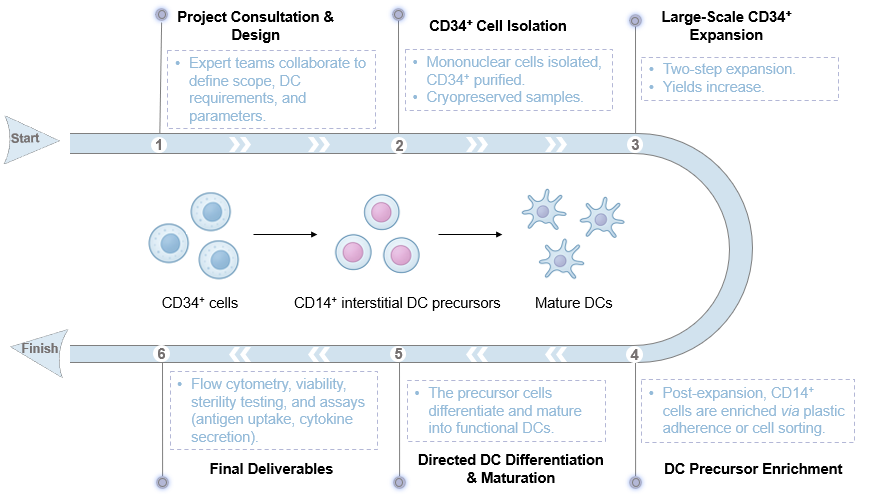
Q2: Can your service generate specific subsets of dendritic cells?
A2: Yes, our proprietary two-step culture system and optimized cytokine cocktails allow for directed differentiation towards specific DC subsets. For instance, our protocols preferentially yield CD14+ interstitial DC precursors, and we can explore customization for other subsets based on your research objectives.
Q3: How do you ensure the quality and functionality of the generated dendritic cells?
A3: We employ a multi-faceted quality control process. This includes stringent phenotypic characterization via flow cytometry for markers. Furthermore, we conduct comprehensive functional assays, including antigen uptake, chemotaxis, and the allogeneic mixed leukocyte reaction, to confirm their potency and effectiveness in immune activation.
Choosing Creative Biolabs for your cord blood CD34+ hematopoietic stem/progenitor cell-derived dendritic cell production service means partnering with a leader in cellular immunotherapy, committed to accelerating your scientific breakthroughs. What sets us apart is our exceptional expertise, cutting-edge technological platforms, and uncompromising dedication to quality.
"Using Creative Biolabs' cord blood CD34+ DC production service in our cancer vaccine research has significantly improved the efficiency of T-cell priming. The DCs generated consistently showed higher expression of co-stimulatory markers and a favorable IL-12/IL-10 ratio compared to our in-house methods, leading to more robust antigen-specific T cell responses." – Dr. A. B*er.
"We transitioned to Creative Biolabs for large-scale production of functional DCs for our preclinical studies, and their service has been exceptional. The two-step culture system consistently yields a high quantity of mature DCs, and their quality control ensures reliable functional potency, addressing our previous scalability challenges with alternative sources."–Dr. S. C*uz.
Ready to unlock the full potential of Cord Blood CD34+-derived Dendritic Cells for your next breakthrough? Our team of scientific experts is eager to discuss your specific project needs, provide detailed technical insights, and offer tailored solutions.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION