This service focuses on the efficient differentiation of highly functional dendritic cells from human peripheral blood mononuclear cells (PBMCs), providing a robust source of DCs for diverse immunological studies and therapeutic applications.
In the intricate landscape of biochemistry and biopharmaceutical development, overcoming challenges like long drug development cycles, difficulties in protein expression and purification, and complex clinical trials is paramount. Creative Biolabs provides cost-effective high-purity in vitro dendritic cell (DC) production services designed to accelerate drug discovery, obtain high-quality recombinant proteins, develop highly specific antibodies, and streamline clinical trial processes through innovative cell differentiation and purification techniques.
Dendritic cells are the most potent antigen-presenting cells (APCs), indispensable for initiating and regulating adaptive immune responses. The current landscape of cell-based immunotherapies, including gene therapy, heavily relies on precisely engineered immune cells. Developing high-purity in vitro DCs is crucial for consistent research outcomes, reliable drug screening, and effective therapeutic applications, minimizing off-target effects and ensuring the specificity required for advanced treatments like cancer vaccines and autoimmune disease therapies.
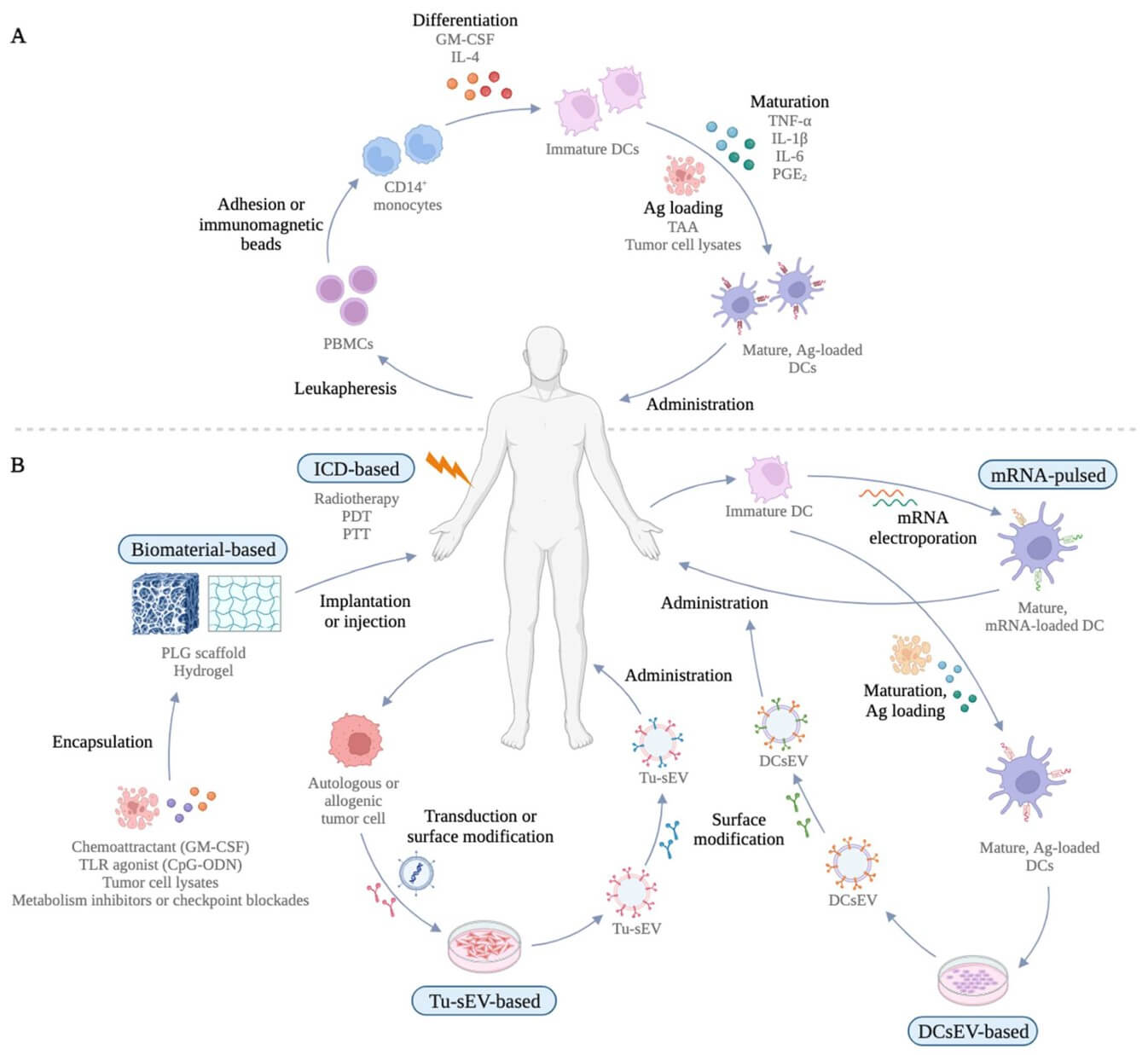 Fig.1 The traditional ex vivo MoDC-derived DC vaccination and new generations of DC vaccines.1
Fig.1 The traditional ex vivo MoDC-derived DC vaccination and new generations of DC vaccines.1
Creative Biolabs' high-purity in vitro dendritic cell production services are founded on the principle of controlled differentiation and precise purification. We start by isolating progenitor cells (e.g., monocytes) from client-provided materials and meticulously guiding their differentiation into immature DCs using optimized cytokine cocktails. These cells are then matured into highly functional antigen-presenting cells, followed by advanced cell sorting technologies like MACS or FACS to achieve exceptionally high purity.
Clients can expect validated, pure DC populations essential for consistent experimental results, robust drug screening, and the development of potent cell-based therapies. The timeline for these services typically ranges from 3 to 6 weeks, depending on project complexity. Our commitment to rigorous quality assurance ensures superior cell viability, purity, and functionality, empowering your projects with reliable cellular components that consistently meet stringent research and developmental needs.
This initial stage involves the meticulous isolation of progenitor cells (e.g., monocytes from PBMCs) from the provided starting materials. The expected outcome is a highly enriched population of precursor cells ready for differentiation.
Specific cytokine cocktails, including GM-CSF and IL-4, are applied to induce the differentiation of progenitor cells into immature dendritic cells over a defined period. This step yields a population of phenotypically immature DCs.
Immature DCs are then treated with maturation-inducing agents (e.g., LPS, TNF-α, or specific danger-associated molecular patterns) to promote their full activation and maturation into professional APCs. The outcome is mature, activated DCs expressing high levels of co-stimulatory molecules and MHC complexes.
Utilizing advanced cell sorting technologies, such as magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS), target DC populations are rigorously purified to achieve exceptionally high purity levels. This crucial step ensures minimal contamination from other cell types, delivering a highly homogeneous DC population.
Comprehensive quality control assessments are performed, including flow cytometry for phenotypic markers (e.g., CD1a, CD14, CD83, CD86, HLA-DR), viability assays, and functional assessments (e.g., antigen uptake, T-cell stimulation capacity). This stage confirms cell identity, purity, viability, and functionality.
Creative Biolabs offers a range of specialized Dendritic Cell Production Services tailored to your specific research and development needs:
This service focuses on the efficient differentiation of highly functional dendritic cells from human peripheral blood mononuclear cells (PBMCs), providing a robust source of DCs for diverse immunological studies and therapeutic applications.
Designed for projects requiring significant quantities of high-purity dendritic cells, this service offers optimized protocols for large-scale manufacturing from PBMCs, ensuring consistent quality and scalability for preclinical and early clinical research.
Leverage the potential of hematopoietic stem and progenitor cells with our service for generating dendritic cells from bone marrow-derived CD34⁺ cells. This offers a unique approach for exploring DC subsets and their developmental pathways.
Explore alternative sources for DC generation with our specialized service utilizing cord blood CD34⁺ hematopoietic stem/progenitor cells. This option is ideal for studies requiring specific developmental characteristics or for pediatric research applications.
Q1: What purity levels can I expect from your DC production services?
A1: We guarantee exceptionally high purity levels, typically exceeding 95% for target DC populations, validated by flow cytometry analysis of specific markers. This ensures minimal cellular contamination for your sensitive experiments.
Q2: Can you differentiate DCs from various starting materials?
A2: Yes, we are proficient in differentiating high-purity DCs from a variety of human and animal sources, including PBMCs, isolated monocytes, and even iPSC lines, catering to diverse research needs.
Q3: How do you ensure the functional activity of the produced DCs?
A3: Beyond phenotypic characterization, we perform rigorous functional assays, such as antigen uptake studies and allogeneic T-cell stimulation assays, to confirm the robust antigen-presenting capabilities and T-cell activating potential of our DCs.
Creative Biolabs' high-purity in vitro dendritic cell (DC) production services provide a robust, reliable, and cost-effective solution for your research and therapeutic development needs. Our commitment to quality, backed by extensive experience and cutting-edge technology, ensures you receive the highest quality cellular components for impactful scientific discoveries. Contact us to get more details.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION