The cytotoxic efficacy of CAR-T cells serves as a core indicator of their tumor-killing capacity. However, their effectiveness in solid tumors remains constrained by several challenges, including insufficient targeting specificity, immunosuppressive tumor microenvironments, and the lack of standardized in vitro evaluation systems. Creative Biolabs' CellRapeutics™ Cytotoxicity Test Services provide professional and comprehensive in vitro assessment solutions for CAR-T cell cytotoxicity. Our service offers robust functional validation data to support CAR-T product optimization, mechanistic studies, and preclinical evaluation, thereby streamlining the development process of immunotherapies.
CAR-T cytotoxicity denotes the genetically redirected capacity of CAR-engineered T lymphocytes to recognize and selectively eliminate target cells bearing the cognate antigen. This targeted cytolysis is executed chiefly through the coordinated release of perforin/granzyme granules, engagement of death-receptor signaling cascades, and the paracrine action of pro-inflammatory cytokines. Rigorous quantification of these cytolytic pathways is essential to evaluate the potency, kinetic profile, and functional stability of a CAR-T product against its neoplastic targets.
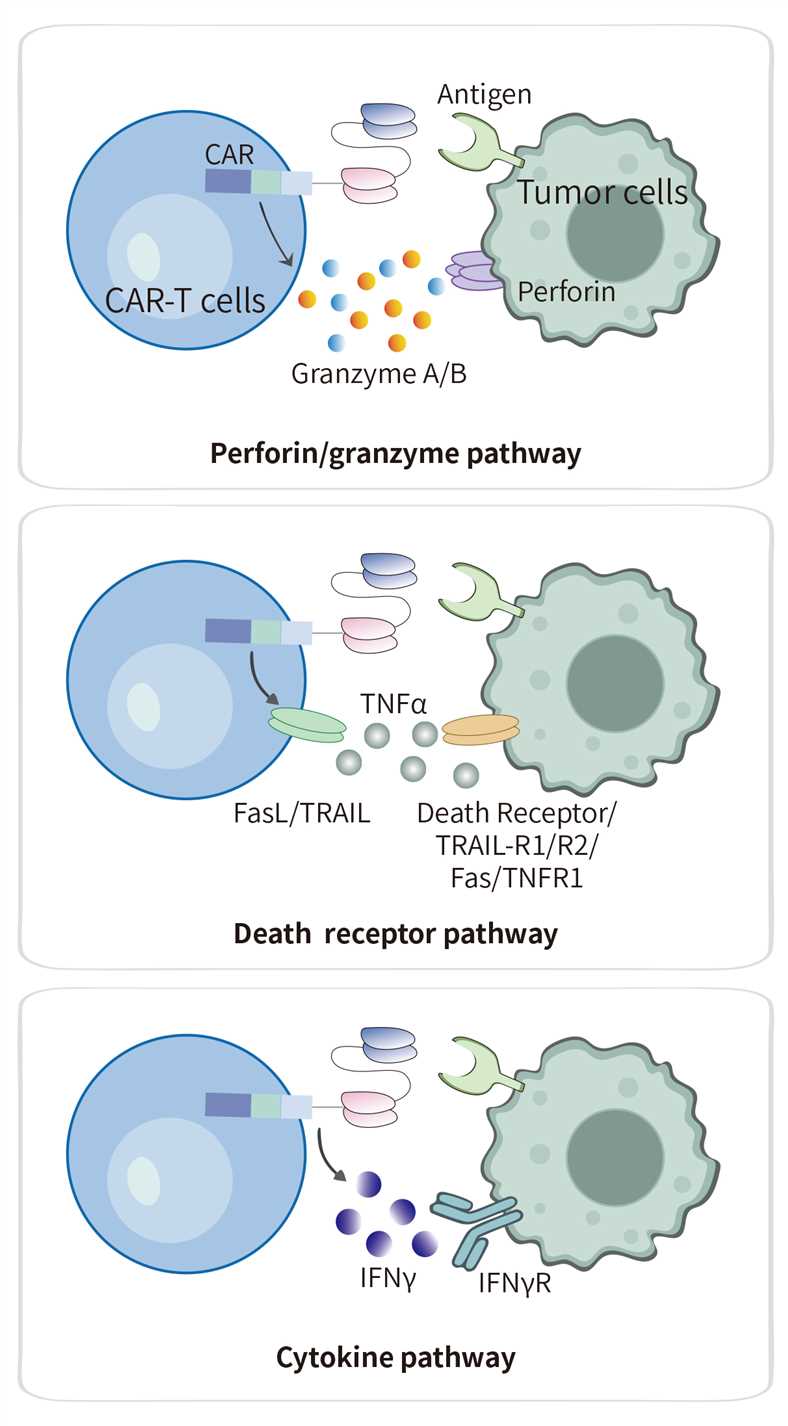 Fig.1 Unveiling the effector mechanisms of CAR-T cell-mediated killing.
Fig.1 Unveiling the effector mechanisms of CAR-T cell-mediated killing.
Creative Biolabs' CellRapeutics™ Cytotoxicity Test Services deliver robust data on cell-mediated cytotoxicity, on-target efficacy, and potential off-target effects, which provides a clear, comprehensive, and dependable assessment of your therapeutic candidates, de-risking your project and paving the way for successful clinical translation. Our services are tailored to your specific project needs, providing the actionable insights you need to make informed decisions.
Driven by a commitment to scientific excellence, our CellRapeutics™ Cytotoxicity Test Services are designed to accelerate your path to discovery. We offer a diverse portfolio of advanced detection platforms to meet your specific research needs.
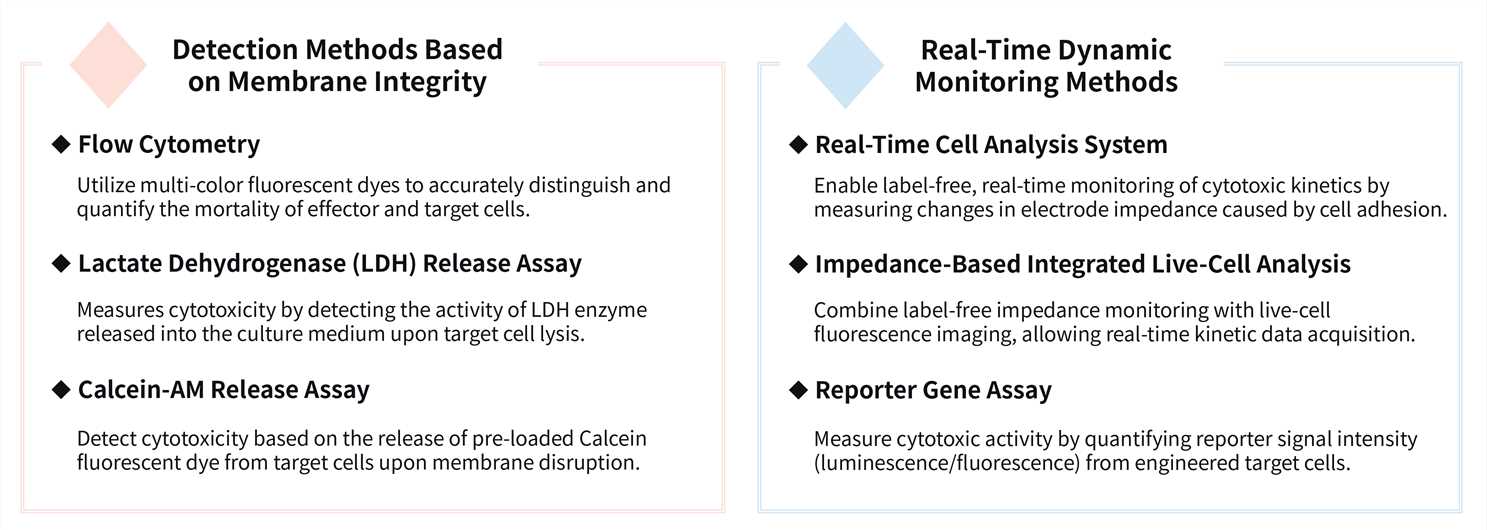
Furthermore, to more accurately model the complex tumor microenvironment and investigate cell-cell or cell-matrix interactions, we offer a 3D Hanging Spheroid Plate-based Cytotoxicity Assay. This platform provides a more physiologically relevant, efficient, and reliable experimental system for evaluating therapeutic efficacy.
Our structured workflow ensures full transparency and operational efficiency, offering a well-defined pathway from initial project setup to the delivery of finalized results. The standard duration for our CellRapeutics™ cytotoxicity testing services is typically between 4 and 8 weeks, varying based on the scope and intricacy of the assay as well as the quantity of therapeutic candidates under assessment.
Required Starting Materials: Prior to project initiation, we kindly request that you provide details regarding the therapeutic agents you intend to use (e.g., CAR-T cells, bispecific antibodies), the target cell lines (such as CD19+ or HER2+ cancer cells), as well as relevant control cells or genetic information (e.g., specific antigen expression data).
Key Steps Involved:
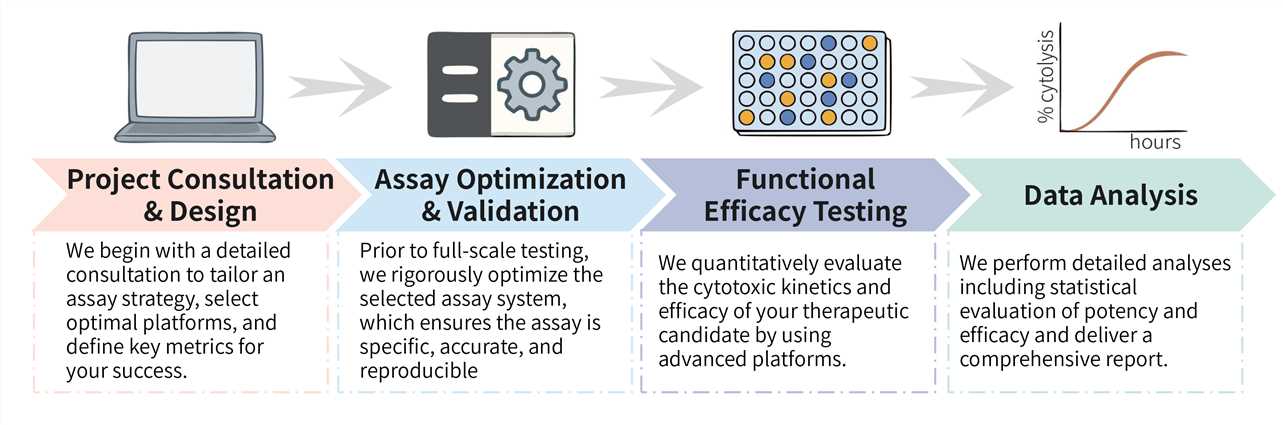
Final Deliverables: You will receive a final report, which includes a comprehensive summary of all findings, raw data files, and an executive summary with clear conclusions.
As we advance a novel CAR-T cell therapy, what evidentiary standards does your validation package satisfy?
Our validation strategy is comprehensive, focusing on key performance characteristics such as linearity, accuracy, precision, and robustness. This includes thorough assessment of intra-assay, inter-assay, and inter-day variability.
We are using a complex co-culture system for our therapeutic candidate. Is this within your capabilities?
Absolutely. Our team has extensive experience designing and executing sophisticated co-culture assays that incorporate multiple cell types to better recapitulate the tumor microenvironment. We encourage you to share details of your model so we can develop a tailored experimental approach that meets your specific requirements.
Creative Biolabs integrates scientific expertise, cutting-edge technologies, and a customer-centric service approach to deliver a comprehensive perspective on therapeutic agent performance. We ensure that your assay results are both robust and translatable, positioning ourselves as strategic partners committed to the success of your projects.
"Using Creative Biolabs' CellRapeutics™ Cytotoxicity Test Services in our research has significantly improved our understanding of the kinetics of CAR-T cell killing. The real-time impedance data provided insights that were simply impossible to get with traditional endpoint assays." Dr. A. Smi*.
"We needed a partner for our therapy development who could guarantee reproducible data. The inter-assay and inter-day precision we received from Creative Biolabs was outstanding. The attention to detail and rigorous validation gave us complete confidence in our data for regulatory submission." Dr. L. Jon*.
"The ability to cross-validate our findings across different platforms, including flow cytometry and live-cell imaging, was a major advantage. Creative Biolabs' approach allowed us to confirm on-target effects while also identifying potential bystander effects, which saved us a lot of time and resources compared to doing it ourselves." Dr. C. Wil*.
Partner with Creative Biolabs for a thorough functional assessment of your cell therapies using our CellRapeutics™ cytotoxicity services. Contact us to align our expertise with your development goals.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION