Choose from standard human triculture models or request disease-specific conditions (e.g., neuroinflammation, tumor-like permeability). Tailoring begins with understanding compound class and transport needs.
The blood-brain barrier (BBB) is a highly selective interface that limits CNS drug delivery. Approximately 98% of small molecules and nearly all large biologics fail to cross the BBB effectively. Leveraging recent breakthroughs in triculture co-culture systems, human iPSC-derived cells, and dynamic microfluidics, in vitro BBB models now offer predictive insights into brain permeability. Are you struggling with high CNS drug attrition, poor BBB predictability, or lack of reliable early-stage screening platforms? Creative Biolabs delivers next-generation BBB permeability assays validated by tight junction markers, transporter activity, and transendothelial electrical resistance (TEER).
We provide customizable, physiologically relevant in vitro BBB assay services for:
Our service delivers high-confidence BBB screening results that drive CNS-targeted drug development forward. With Creative Biolabs, clients receive:
Learn How We Can Support You – Schedule a Consultation
Creative Biolabs integrates advanced BBB modeling strategies designed to closely mimic in vivo physiological and transport conditions:
These approaches ensure translatable, reproducible, and cost-effective solutions for pharmaceutical and academic CNS research.
Choose from standard human triculture models or request disease-specific conditions (e.g., neuroinflammation, tumor-like permeability). Tailoring begins with understanding compound class and transport needs.
Cells are seeded in Transwell or microfluidic chambers. Validation includes TEER monitoring, immunostaining of tight junction markers (e.g., ZO‑1, Occludin), and permeability to standard probes.
Your compound is added to the apical side. Aliquots are sampled at defined intervals from the basolateral chamber for concentration analysis.
Papp is calculated; optional studies evaluate efflux behavior using known transporter inhibitors to dissect mechanisms.
Confocal microscopy or HCS imaging evaluates compound localization and barrier disruption effects.
Results are compiled into a tailored technical report including permeability kinetics, barrier integrity graphs, and expert recommendations for next steps.
Built with iPSC-derived cells, our models replicate the neurovascular unit with high TEER values and strong expression of tight junction and transporter proteins, offering reliable BBB permeability assessment.
Choose from Transwell, microfluidic chip, or 3D spheroid models tailored to different throughput and compound classes, supporting everything from screening to mechanistic studies.
Simulate disease-relevant conditions like inflammation or hypoxia to assess compound behavior in compromised BBB environments such as tumors or neurodegeneration.
Supports 96- and 384-well formats and automated workflows, enabling fast screening of large compound libraries during early-phase drug discovery.
Discover the Creative Biolabs Difference – Request Your Quote Today
Drug permeability results reveal distinct transport characteristics across in vitro blood-brain barrier models established with human iPSC-derived brain microvascular endothelial cells. Compounds with high permeability, such as propranolol and caffeine, efficiently crossed the barrier, while low-permeability compounds like atenolol and inulin showed restricted passage. The data underscore the model's ability to discriminate between passive diffusion and restricted transport, aligning closely with known in vivo behavior. This highlights its utility for early-stage CNS drug screening, allowing accurate classification of compounds based on their BBB penetration potential.
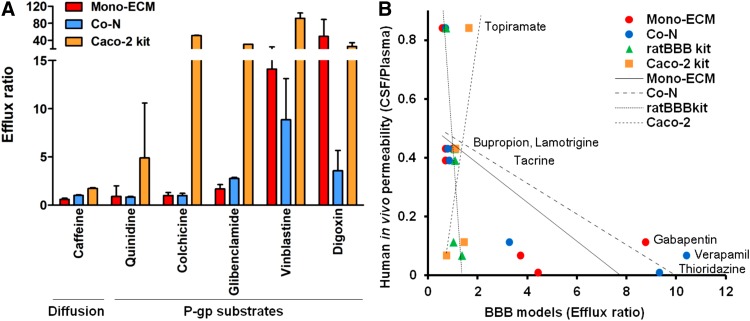 Fig.1 Assessment of drug permeability.1
Fig.1 Assessment of drug permeability.1
Can your in vitro BBB model predict drug permeability for large biologics?
Yes. We offer transcytosis-compatible models capable of assessing antibody and peptide-based therapeutics using advanced imaging and receptor-mediated transport assays.
What is the minimum sample volume or quantity needed for a full assay run?
Typically, we require 100–500 µL of a 10–50 µM test compound, depending on the assay format and time points.
Do your assays include transporter activity evaluation?
Absolutely. Our standard service includes evaluation of P-gp and BCRP activity, with optional inhibition assays to study transporter-mediated effects.
Creative Biolabs also offers several complementary services to enhance your BBB permeability testing project.
Our in vitro cytotoxicity analysis service enables precise evaluation of compound-induced cellular toxicity using standardized assays across multiple cell lines and detection methods. It supports early safety screening by providing dose-response profiles and mechanistic insights to guide preclinical decision-making.
Creative Biolabs' Immune Genotyping Services offer comprehensive analysis of immune-related genetic variations, including HLA typing, TCR/BCR repertoire profiling, and immune SNP detection. These services empower biomarker discovery, patient stratification, and the development of personalized immunotherapies.
Creative Biolabs provides cutting-edge, human-relevant BBB permeability assessment solutions tailored to support neuropharmaceutical innovation. From discovery to preclinical studies, we help you reduce risk, save time, and enhance translatability.
Contact Our Team for More Information and to Discuss Your Project
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION