Are you currently facing high inter-laboratory variability, concordance failure in multi-site clinical trials, or challenges obtaining precise individual cell functional data for immunotherapy candidates? Creative Biolabs' Intracellular Cytokine Staining (ICS) Analysis Service helps you streamline clinical trial processes and accelerate drug discovery by delivering highly precise and reproducible T cell functional profiling. We achieve this through rigorous, harmonized workflow and expert, artifact-mitigating protocols validated across global proficiency panels.
Cytokine Release Syndrome (CRS) is the most common and potentially life-threatening adverse reaction associated with CAR-T cell therapy, with its incidence and severity critically influencing both therapeutic success and patient safety. Precise monitoring of dynamic cytokine changes in patients is essential for the early prediction, grading, and timely intervention of CRS, serving as a pivotal step to maximize therapeutic benefits and minimize risks. Consequently, ICS analysis, a technique capable of functionally profiling individual immune cells, provides an indispensable tool for elucidating the driving mechanisms of CRS and tracing cytokine sources.
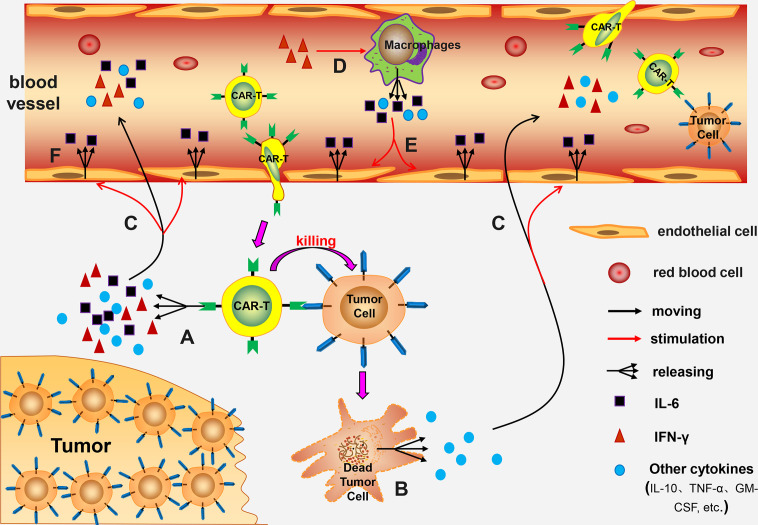 Fig.1 The cytokine storm: mechanisms and implications in immunotherapy.1
Fig.1 The cytokine storm: mechanisms and implications in immunotherapy.1
Creative Biolabs' Intracellular Cytokine Staining (ICS) Analysis Service provides accurate and reliable functional data for vaccine, infectious disease, and oncology programs. The complexity of ICS, which involves a multi-step protocol (stimulation, blocking, fixation, permeabilization) and detailed flow cytometric data analysis, is a major source of variability. Our service mitigates these risks, delivering data that is robust and fit for regulatory submission.
Our ICS analysis service delivers robust, high-resolution functional immune-profiling through a fully optimized workflow encompassing experimental design, sample processing, and data acquisition.
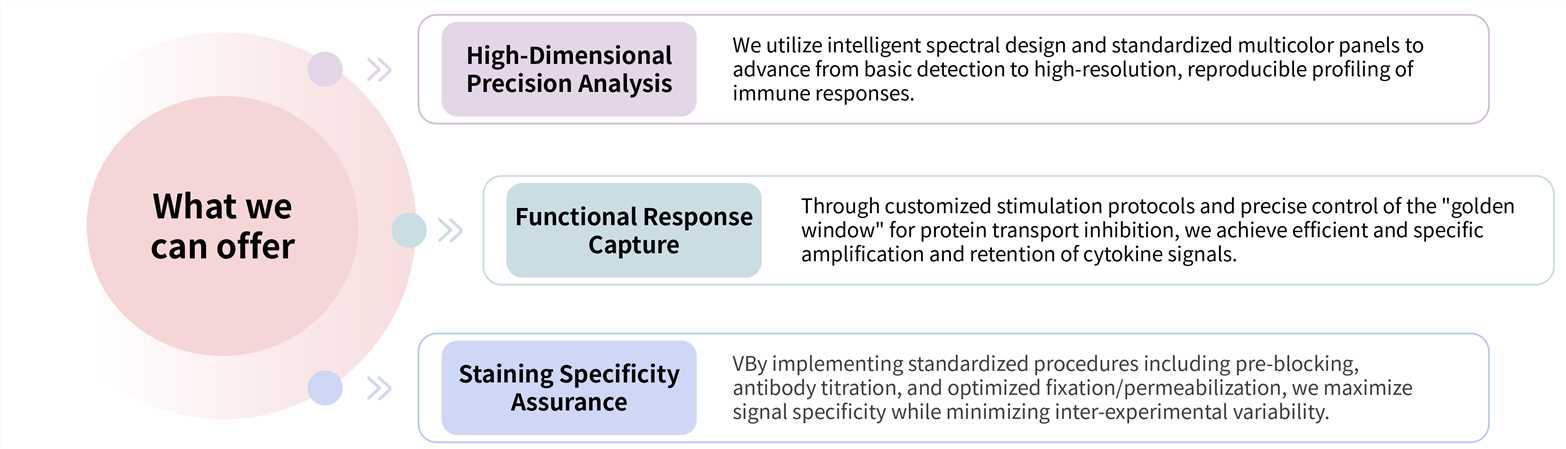
Required starting materials:
Key Steps Involved:
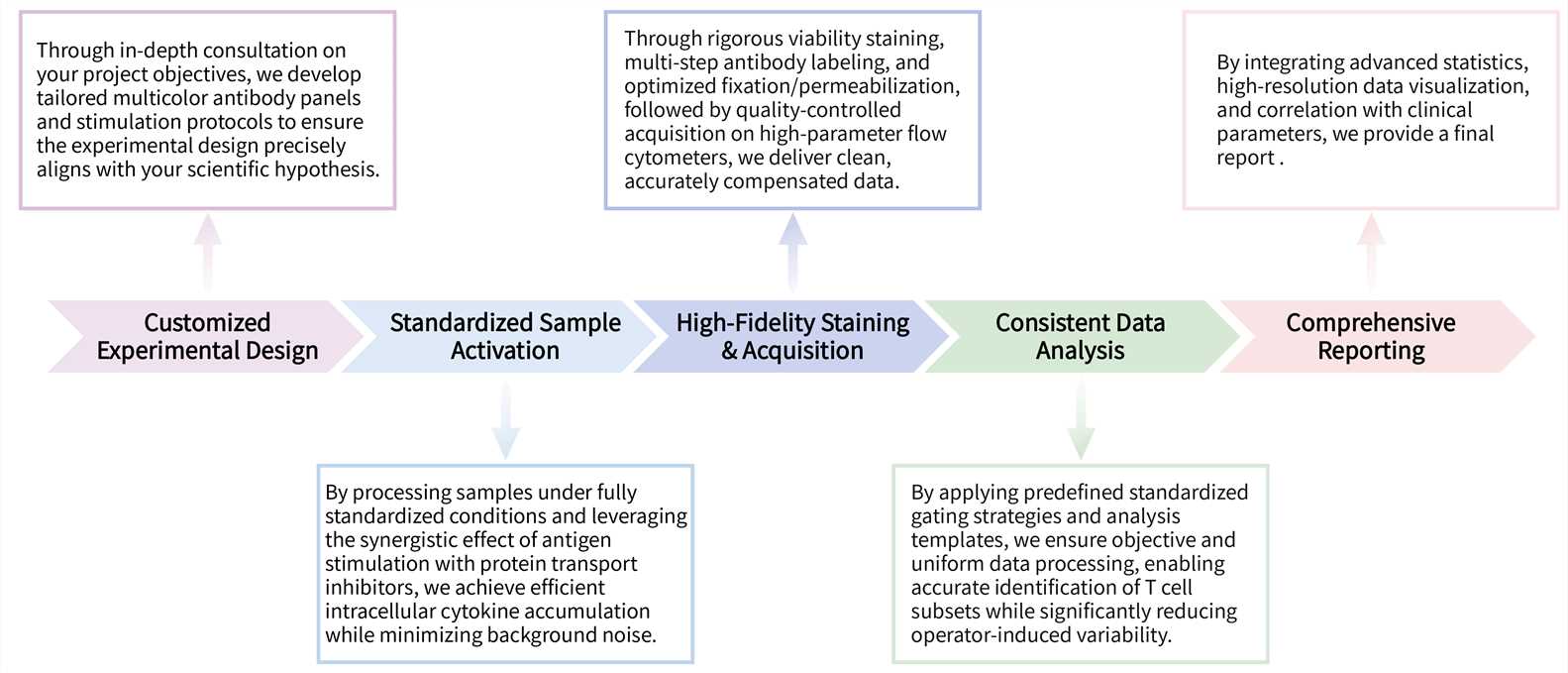
Final Deliverables:
How do you ensure data comparability across my multi-site clinical trial samples?
We use proprietary, globally harmonized workflow including centralized processing, pre-titrated antibody panels, and most critically, a standardized, validated gating strategy that eliminates the high variability caused by subjective data analysis in different labs.
What is the maximum number of cytokine targets and phenotypic markers I can analyze in a single assay?
Our advanced flow cytometry platform allows for high-parameter analysis, supporting complex panels up to 18 colors. This enables the simultaneous detection of 8+ distinct intracellular cytokine/functional markers alongside detailed phenotypic subsets. Contact us to discuss your specific panel needs.
As your trusted partner in high-fidelity immune-monitoring, Creative Biolabs delivers gold-standard ICS Analysis. Our service replaces experimental variability with rigorous standardization and deep scientific insight, transforming your data into clinically relevant, actionable results to accelerate vaccine and immunotherapy programs.
"Using Creative Biolabs' Intracellular Cytokine Staining (ICS) Analysis Service in our multi-site vaccine study has significantly improved the concordance and reproducibility of our IFN-γ and IL-2 T-cell responses, allowing us to confidently pool data from different geographies."— Dr. Js M. Fr.
"The team at Creative Biolabs successfully identified and corrected a subtle, confounding artifact in our crude tumor digest samples. Their protocols prevented the H2O2-mediated T cell death that was masking true antigen-specific activation in our initial analysis, giving us accurate data on CD8+ T cell function."— Prof. Er A. Ts.
"We utilize Creative Biolabs' ICS service for a detailed look at the quality of our immune response. Their expert multi-color panel design allowed us to accurately measure five distinct functional T cell subsets, giving us the functional data needed to differentiate our lead candidate from competitors."— De P. An.
For a detailed consultation on your project needs, custom panel development, or to request a technical validation package, please contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION