In recent years, CAR-NK cell therapy has achieved impressive clinical results and proved to be a powerful immune effector in clinical trials. However, unlike CAR-T cells, lentiviral vectors have proven to be an effective method for genetic modification in CAR-T cells, while lentiviral transduction is less efficient in CAR-NK cells. In addition, the potential for insertional mutagenesis and genotoxicity of viral vectors leads to safety issues, and its cost and regulatory requirements for rapid and extensive clinical translation become obstacles.
To achieve high efficient transfection and strong tumor cell killing of CAR-NK cells in adoptive immunotherapy, Creative Biolabs provides multiple non-viral transfection methods for efficient genetic modification of NK cells, including Sleeping Beauty (SB) transposon vectors, piggyBac (PB) system, and mRNA. These non-viral genetic engineering methods create stable genomic integration of target genomic material.
At Creative Biolabs, we provide customized vector design and comprehensive services for non-viral vector systems, including SB vectors, PB vectors, and nucleic acid vectors, such as plasmid DNA and mRNA. These vectors can be highly transfected by multiple non-viral delivery technologies, including liposome formulations, nanoparticles, advanced electroporation methods (such as nuclear transfection), and cell penetrating peptides.
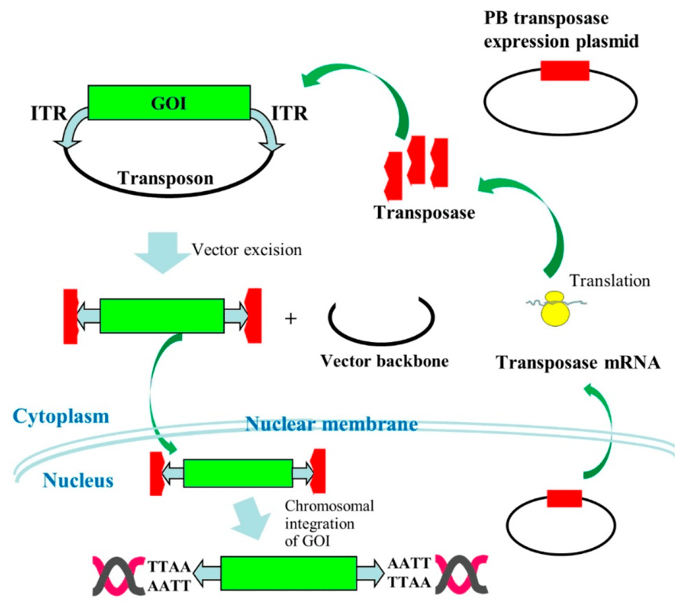
Fig.1 Mechanism of PB-mediated transposition.1
Non-viral vectors show better safety and reduced production costs, however, they cannot provide long-term cellular uptake, nuclear maintenance, and "on-spot" target delivery. Notably, a class of non-viral vectors, transposon-based gene delivery systems, not only has the advantages of integrating viral vectors (e.g, stable chromosomal integration and durable transgene expression), but also has the characteristics of non-viral delivery systems (e.g, low Immunogenicity, enhanced safety, and reduced GMP manufacturing cost), which are ideal choices for non-viral systems.
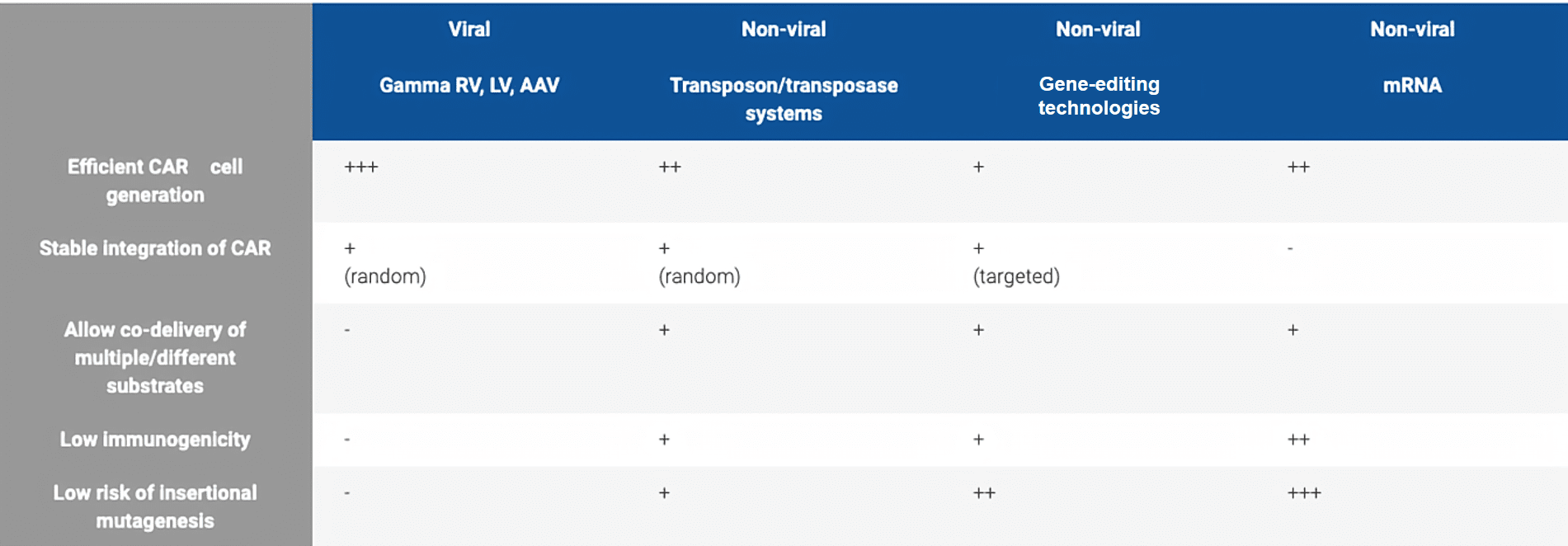
Fig.2 Comparison of viral and non-viral delivery modes for CAR cell generation.
Notably, as the leading cell therapeutics biotech that provides cell therapy related services, Creative Biolabs provides one-stop services for CAR-NK therapy development, including but not limited to:
If you are interested in our services, please send an email to contact us, and our team will get back to you as soon as possible.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION