All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Chimeric Antigen Receptor (CAR) T-cell therapy stands at the frontier of modern oncology, representing one of the most promising treatment modalities for cancer, particularly hematological malignancies. The development of CAR T-cell therapies requires stringent manufacturing protocols to ensure product safety, efficacy, and reproducibility.
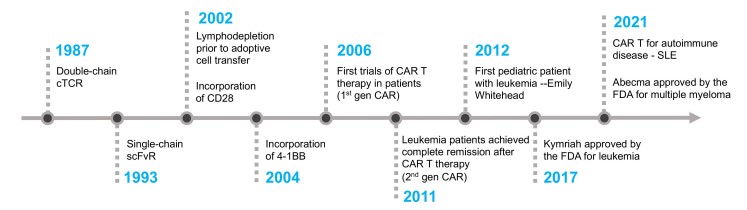 Fig.1 The key milestones of CAR T cell development.1
Fig.1 The key milestones of CAR T cell development.1
The clinical success witnessed with CAR T-cell therapies emphasizes the critical need for GMP-compliant production processes. These therapies have been transformative, notably in treating B-cell lineage malignancies. The complexity of CAR T-cell manufacturing necessitates an elaborate infrastructure compliant with GMP regulations to ensure the production of safe, potent, and effective therapeutic agents.
GMP compliance ensures that CAR T-cells are manufactured under controlled and auditable conditions, which are crucial for the consistency and safety of therapeutic outcomes. In-house production facilities must navigate regulatory landscapes, grapple with the infrastructural design to prevent contamination, and address potential manufacturing failures. Statistics show a failure rate of 2-14% in CAR T-cell production, often due to insufficient cell yields or contamination issues, underscoring the need for robust GMP processes.
With extensive experience in cellular and gene therapy product development, Creative Biolabs offers a comprehensive suite of GMP-level services, ranging from plasmid manufacture to T-cell production.
Utilizing bioreactor technologies, Creative Biolabs enables large-scale plasmid production, achieving yields of several hundred milligrams per liter within short timeframes. This scalable approach overcomes the limitations associated with traditional flask cultures, which typically fail to meet the demands of large-scale CAR T-cell production.
The role of viruses in gene transfection is indispensable in CAR T-cell development. Creative Biolabs employs a proprietary HEK cell line to facilitate high-efficiency lentivirus packaging. This capability supports efficient transfection processes, crucial for the stable expression of CAR constructs.
The GMP CAR-T cell production at Creative Biolabs pivots on the company's self-developed large-scale production technologies that ensure product purity, safety, and potency. Such refined processes cater to the growing clinical demand and allow for streamlined CAR T-cell therapy manufacturing.
Creative Biolabs takes pride in a diverse portfolio of GMP-approved CAR-T products designed to meet varied therapeutic needs. Our offerings include:
Comprehensive GMP grade vectors that are tailed for precision in targeting specific cancer antigens.
High-titer lentiviral particles crafted to facilitate efficient genetic modification of T cells.
A wide variety of reagents are used in GMP production, such as GMP-grade cytokines for the culture and activation of cells of specific origin.
Creative Biolabs ensures high-quality, consistent products that are indispensable for effective CAR T-cell therapy development. The benefits include:
Q1: Why is GMP compliance critical in CAR T-cell development?
A1: GMP compliance is essential to ensuring that CAR T-cell products are safe, effective, and consistent. It governs all aspects of production, from facility design to process validation and quality control.
Q2: How does Creative Biolabs ensure the quality of its GMP CAR-T products?
A2: Quality is ensured through a combination of advanced production technology, rigorous quality control testing, and adherence to proven GMP protocols.
With this in-depth exploration, we highlight the centrality of GMP products in advancing CAR T-cell therapies, a field brimming with potential to redefine cancer treatment paradigms. Through the pioneering efforts of Creative Biolabs, the future of cellular therapies promises unprecedented progress in the fight against cancer.
Reference
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION