We engineer logic-gated CARs implementing AND/OR/NOT signaling. Our services include design and validation, addressing off-target effects, improving tumor selectivity, and enabling safer, more precise cellular therapies.
T cell engineering with an antigen-binding antibody fused to extracellular domain of TCR subunits is a promising cell therapy against solid tumors. This kind of therapy has demonstrated encouraging anti-cancer results in pre-clinical trials. As a leading end-to-end CRO, Creative Biolabs provides talented scientists and cutting-edge technologies to help our clients develop TCR-fused Antigen Binding Receptor (TCR-ABR) CAR-engineered T cells.
T cells play an important role in protecting hosts from pathogens and tumors. Their functions are based on the T-cell receptor (TCR)-CD3 complex, in which TCRαβ heterodimer noncovalently combines with the constant CD3 dimers CD3εγ, CD3εδ, and CD3ζζ. The TCRαβ chains and each subunit of the CD3ϵγ and CD3ϵδ heterodimers comprise an extracellular domain, connecting peptide, transmembrane (TM) domain, and a short cytoplasmic tail. Each of the CD3 ϵ, γ, δ, and ζ chains contains immunoreceptor tyrosine-based activation motifs (ITAMs) that can be phosphorylated to initiate the T-cell signaling transduction pathway. Following TCR/pMHC binding, the CD3 molecules transmit activation signals to the T cell.
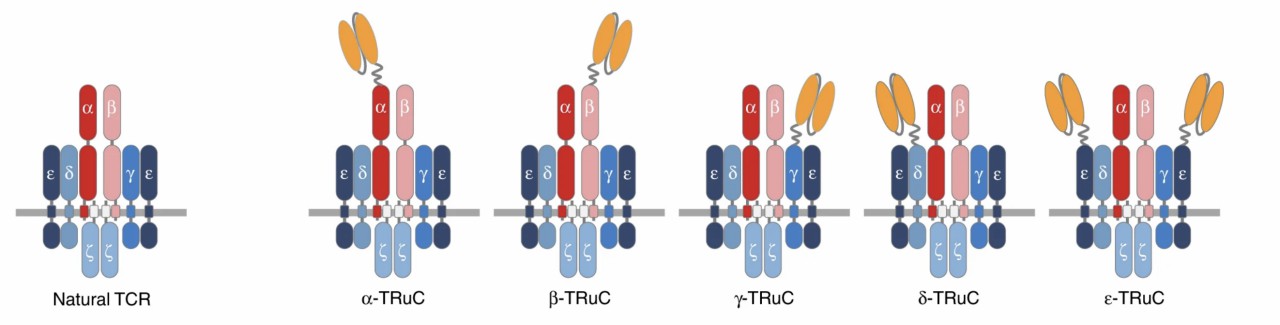 Fig.1 The natural TCR complex and five TRuC-containing TCR complexes.1
Fig.1 The natural TCR complex and five TRuC-containing TCR complexes.1
Antigen-binding domains fused to TCR subunits have been shown to efficiently initiate an intact TCR complex reaction against tumor surface antigens. These components are also named synthetic T cell receptor fusion constructs (TRuCs). The scFv used to generate CAR-T cells or single domain antibodies can be fused to the extracellular N-termini of TCRα, TCRβ, CD3γ, CD3δ, or CD3ε subunits, resulting in the activation of target-specific TRuC-T cells. TRuC-T cells are as effective as second-generation CAR-T cells at killing tumor cells. In some tumor models, TRuC-T cells exhibit potent anti-tumor activity. In some other models, TRuC-T cells outperform respective CAR-T cells. TRuC-T cells have been shown to use the entire TCR complex's signaling capacity in an HLA-independent manner.
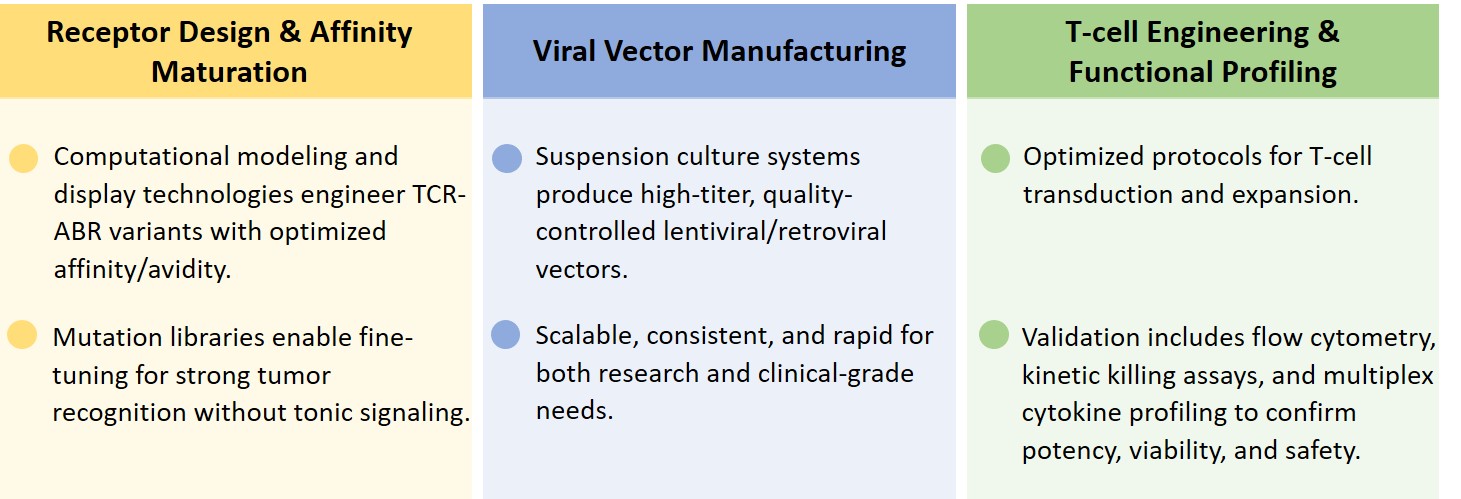
 Fig.2 Advantages of the TCR-ABR-CART cells.
Fig.2 Advantages of the TCR-ABR-CART cells.
Creative Biolabs has built an end-to-end platform to support our customers' TCR-ABR-CART cell development programs from discovery to pre-clinical studies, combining rich experience with a deep knowledge of advanced technologies in the development of cell therapeutics. We provide TCR-fused Antigen Binding Receptor (TCR-ABR) CAR construction and TCR-ABR-CART cell production services. The scFv sequence will be determined according to the specific targets. The fusion subunit of TCR will be determined based on your specific requirements (TCRα, TCRβ, CD3γ, CD3δ, or CD3ε). The TCR-fused Antigen Binding Receptor (TCR-ABR) CAR genes will be introduced into T cells using lentivirus transfection or other approaches.
Creative Biolabs offers high-quality, innovative products and solutions to help you accelerate TCR-ABR-CART cell therapy discovery and development projects. We are committed to understanding and meeting the quality needs and expectations of our customers. Please contact us to learn more.
Creative Biolabs utilizes integrated, proprietary technology platforms to ensure the successful design, construction, and validation of your TCR-ABR CAR project. These platforms streamline the workflow from target identification to functional T-cell validation.
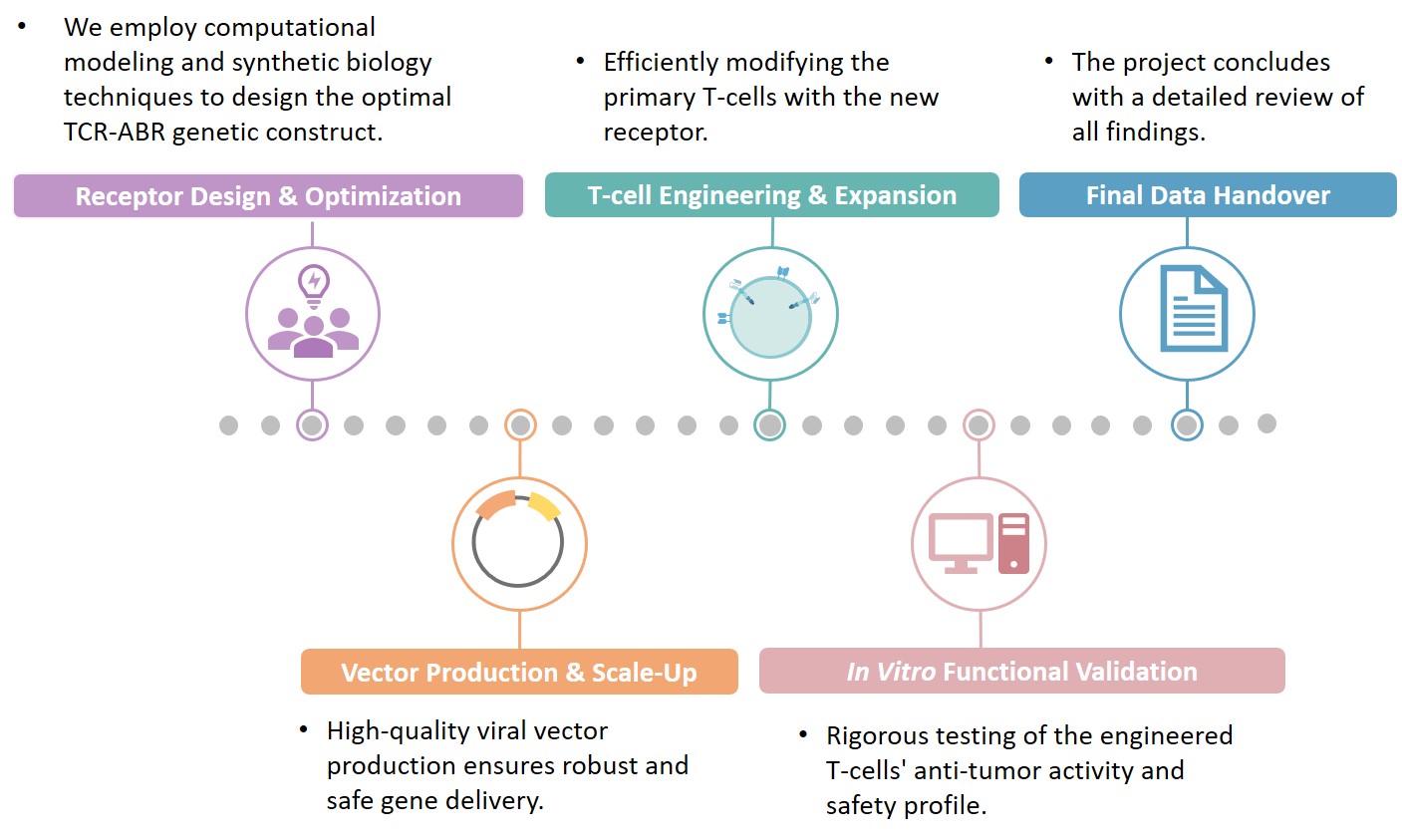
Final deliverables include a comprehensive data report, purified high-titer viral vector stock, and validated standard operating procedures for T-cell transduction and expansion.
Request information now for thorough specifications and customize your service package.
What is the primary advantage of TCR-ABR over a standard second-generation CAR?
The main advantage is the ability to target intracellular tumor-associated antigens presented on the cell surface via MHC molecules. Traditional CARs are limited to surface-expressed antigens, whereas the TCR-ABR expands the target universe significantly, which is critical for tackling solid tumors with poor surface antigen markers.
How does Creative Biolabs ensure the safety and low off-target toxicity of the engineered TCR-ABR T-cells?
We prioritize specificity during the design phase by optimizing the TCR domain's affinity. Post-engineering, we employ rigorous receptor validation assays and cross-reactivity screening against a panel of healthy human cells. This functional validation minimizes the risk of on-target, off-tumor toxicity.
We engineer logic-gated CARs implementing AND/OR/NOT signaling. Our services include design and validation, addressing off-target effects, improving tumor selectivity, and enabling safer, more precise cellular therapies.
Our team develops FR806 fusion switch CARs with drug-regulated control. Services cover construct design and validation, addressing uncontrolled activation and improving treatment safety through reversible modulation.
We construct KIR-based CARs leveraging innate immune signaling. Our services encompass design, optimization, and testing, addressing signaling limitations and enhancing immune function for improved cancer therapy.
Creative Biolabs is your dedicated partner in navigating the complexities of next-generation cellular therapies. Our TCR-ABR CAR construction service combines twenty years of scientific expertise with cutting-edge, proprietary platforms to deliver highly specific, potent, and persistent T-cell products optimized for solid tumor challenges. We provide the essential technology and data required to confidently advance your immunotherapy candidate from concept to clinic. Ready to engineer the future of cancer immunotherapy? Reach out to our team today for a confidential discussion about your specific research goals and technical requirements.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION