Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are well-recognized complications of CAR-T therapy, a distinct entity termed tumor inflammation-associated neurotoxicity (TIAN) has emerged as a critical consideration, particularly in the context of CNS tumors. TIAN is characterized by localized neurotoxicity arising from inflammation within the CNS tumor microenvironment. This localized inflammation can manifest in two primary forms: Type 1 TIAN, driven by peritumoral edema leading to increased intracranial pressure and potentially life-threatening herniation; and Type 2 TIAN, resulting from neural-immune interactions that disrupt local neural function, even in the absence of significant edema. This distinction from the more systemic toxicities of CRS and ICANS is paramount for accurate diagnosis, appropriate management, and improved patient outcomes. The unique pathophysiology of TIAN, often occurring in the complex setting of pre-existing CNS pathology, necessitates specialized diagnostic and monitoring approaches.
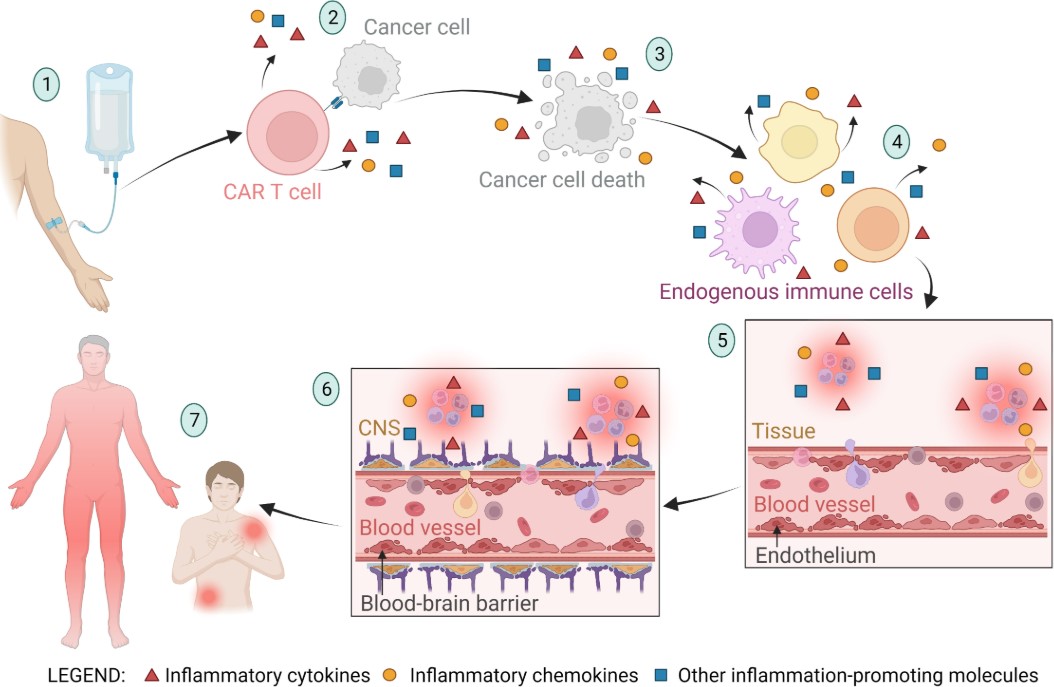 Fig.1 A schematic of CRS and ICANS in CART therapy.1
Fig.1 A schematic of CRS and ICANS in CART therapy.1
Specific inflammatory markers implicated in CAR-T-related neurotoxicity, and potentially relevant to TIAN, include:
Creative Biolabs offers a comprehensive suite of services designed to support the development and implementation of inflammatory marker screening strategies for TIAN management. Our expertise in cytokine and biomarker analysis, coupled with our state-of-the-art laboratory facilities, positions us as a valuable partner for researchers, clinicians, and pharmaceutical companies working in the field of CAR-T cell therapy.
Creative Biolabs' services encompass a wide range of analytical techniques, including:
Simultaneous quantification of multiple cytokines and inflammatory markers in a single sample, enabling efficient and cost-effective analysis.
High-sensitivity and high-specificity assays for the detection and quantification of individual biomarkers.
Measurement of intracellular and cell-surface markers, including cytokine production by specific immune cell populations.
Measurement of gene expression levels of inflammatory mediators, providing insights into the underlying biological processes.
Development of novel assays tailored to specific research or clinical needs, including the identification and validation of new TIAN specific biomarkers.
Creative Biolabs offers several key advantages that make us an ideal partner for TIAN related research and clinical applications:
Q1: What sample types can Creative Biolabs analyze for TIAN related inflammatory markers?
A1: Creative Biolabs can analyze a variety of sample types, including serum, plasma, cerebrospinal fluid (CSF), and tissue specimens.
Q2: Can Creative Biolabs develop custom assays for novel TIAN biomarkers?
A2: Yes, Creative Biolabs has expertise in custom assay development and can work with clients to identify, validate, and develop assays for new TIAN specific biomarkers.
Q3: Does Creative Biolabs adhere to regulatory standards?
A3: Yes, Creative Biolabs adheres to relevant regulatory guidelines and standards to ensure the reliability and validity of the data for research and clinical applications.
To learn more about Creative Biolabs' TIAN related inflammatory marker screening services and how we can support your research or clinical needs, please contact us today.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION