Chimeric antigen receptor T-cell (CAR-T) therapy has revolutionized the treatment of hematologic malignancies and demonstrated remarkable clinical response rates for patients with relapsed or refractory cancers. However, despite its efficacy, CAR-T cell therapy is associated with a unique spectrum of toxicities that can significantly impact patient outcomes. Among these, neurological complications pose a serious challenge. While immune effector cell-associated neurotoxicity syndrome (ICANS) and cytokine release syndrome (CRS) are the most recognized neurological toxicities, emerging evidence highlights the existence of distinct CAR-T-related neurotoxicities. One such entity is Tumor Inflammation-Associated Neurotoxicity (TIAN), characterized by localized tumor inflammation and associated neurotoxicity. TIAN represents a specific neurotoxic effect distinct from ICANS, underscoring the nuanced nature of CAR-T therapy-related neurological complications and the need for specific diagnostic and monitoring tools.
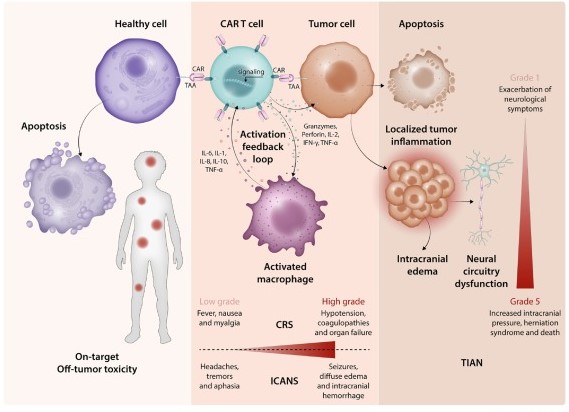 Fig.1 CART therapy-associated toxicities.1
Fig.1 CART therapy-associated toxicities.1
Creative Biolabs recognizes the critical need for proactive management of CAR-T therapy-related toxicities. Our TIAN-related Early Biomarker Screening Service is designed to address this challenge by providing clinicians with the tools to identify and monitor patients at risk for TIAN. This service empowers healthcare providers to implement timely interventions, potentially preventing or minimizing the severity of neurological complications.
Our TIAN-related Early Biomarker Screening Service focuses on the analysis of specific biomarkers in patients' biological samples, such as blood or cerebrospinal fluid. These biomarkers serve as indicators of an elevated risk of developing TIAN. Our featured services include:
This service focuses on quantifying key cytokines involved in the inflammatory processes associated with TIAN. By monitoring changes in cytokine levels, we can identify patients at increased risk and provide early warning signals.
This service analyzes a broader range of inflammatory markers beyond cytokines, including chemokines and other mediators. This comprehensive solution offers a more detailed understanding to the inflammatory cascade, and helps to improve risk stratification.
This service measures specific markers of neuronal injury and dysfunction. These markers can provide direct evidence of neurological damage and help to assess the severity of TIAN.
Q1: What is the clinical significance of TIAN?
A1: TIAN is a neurotoxic complication of CAR-T therapy that can lead to severe neurological dysfunction and increased mortality. Early detection and intervention are essential to improve patient outcomes.
Q2: How do you ensure the accuracy of the testing?
A2: Creative Biolabs adheres to strict quality control protocols, including:
Regular calibration and maintenance of laboratory equipment.
Use of validated assays and reagents.
Proficiency testing and participation in quality assurance programs.
Stringent review of all test results by qualified personnel.
Creative Biolabs offers a comprehensive portfolio of services to support CAR-T therapy, including:
For more information about Creative Biolabs' TIAN-related Early Biomarker Screening Service and our other CAR-T therapy support solutions, please contact our team of experts. We are committed to cooperate with clinicians and researchers, to improve the safety and efficacy of CAR - T treatments.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION