The Safety Frontier-Assessing and Managing Risks of Viral Vectors in Gene Therapy
Viral Vectors for gene therapy
- Adenovirus Vectors
The genome of adenoviruses spans 34 kb to 43 kb in size and uses alternative splicing to produce encoded genes in both the sense and antisense orientations with its double-stranded DNA. The widespread issue with adenoviral vectors arises from the fact that their particles trigger cellular inflammatory reactions instead of remaining inert like Adeno-associated virus vector (AAV) virions. Adenoviral vectors demonstrate substantial benefits compared to other viral gene delivery systems. The primary effectiveness of adenovirus vector as a means for in vivo gene delivery arises from the expression of primary adenovirus receptors and secondary integrin receptors on most human cells. Human cells can be readily infected by adenovirus vectors which leads to robust transgene expression levels. Gutless adenoviral vectors enable us to avoid immune responses against adenoviral vectors. The adenovirus vector stands as one of three key viral vector systems in gene therapy research alongside AAV and lenti/retrovirus vector systems.
- Adeno-Associated Virus Vectors
AAVs belong to the nonpathogenic parvovirus group and feature a linear single-stranded DNA genome that measures roughly 4.7 kilobases with two 145 nucleotide-long inverted terminal repeats at each end. AVV stands as one of the principal vectors for gene therapy applications because it maintains long-lasting and effective transgene expression across multiple cell types throughout several tissues including the liver, muscle tissue, retina and the central nervous system (CNS). The use of AAV vectors presents certain limitations. The existing immunity against human AAV vectors resembles that against AV while integration into the host genome remains random when it does happen which may accidentally trigger or suppress the expression of native genes.
- Lentivirus Vectors
The genetic structure of lentiviruses which belong to retrovirus type is made up of single-stranded positive-sense RNA that turns into DNA through transcription before it integrates into the host genome to produce a lasting infection. The majority of lentiviral vectors originate from HIV-1 and maintain the ability to integrate their genetic material into the genomes of host cells. The popularity of lentiviral vectors in clinical settings stems from their superior capacity to deliver genes into nonproliferating or slowly dividing cells including CD34+ stem cells. Researchers have successfully applied lentiviral vector-mediated gene transfer to CD34+ HSCs for treating genetic conditions such as β-thalassemia and X-linked adrenoleukodystrophy alongside metachromatic leukodystrophy and Wiskott-Aldrich Syndrome. Recent clinical trials implemented third-generation self-inactivating lentiviral vectors to deliver therapeutic genes into hematopoietic stem cells for primary immunodeficiencies and hemoglobinopathy treatment. Scientists use lentiviral vectors to modify T cells by inserting genes that allow them to recognize and attack cancer cells through either chimeric antigen receptors (CARs) or cloned T-cell receptors.
Types of Safety Risks Associated with Viral Vectors
- Immunogenicity and Inflammation
Gene therapy's efficacy and safety can be affected by both innate and adaptive immune responses triggered by viral vectors. The use of adenovirus vectors initiates a strong innate immune reaction that results in vascular endothelial cell activation along with inflammatory cytokine production and macrophage cell death. The robust immune reaction triggers substantial inflammation which compromises therapy effectiveness. AAV vectors produce a weaker and short-lived innate immune response but they retain the ability to trigger TLR9 signaling pathways and stimulate CD8+ T cell activity.
Clinical trials have shown that high doses of adenovirus vectors can trigger severe systemic inflammatory reactions which result in multiple organ failure complications. A patient who received adenovirus-based gene therapy experienced severe systemic inflammatory response syndrome (SIRS), demonstrating the importance of managing immune responses during treatment. Preclinical research has demonstrated that AAV vectors trigger neutralizing antibodies (NAbs) and CD8+ T cell reactions that decrease the effectiveness of multiple dosing schedules.
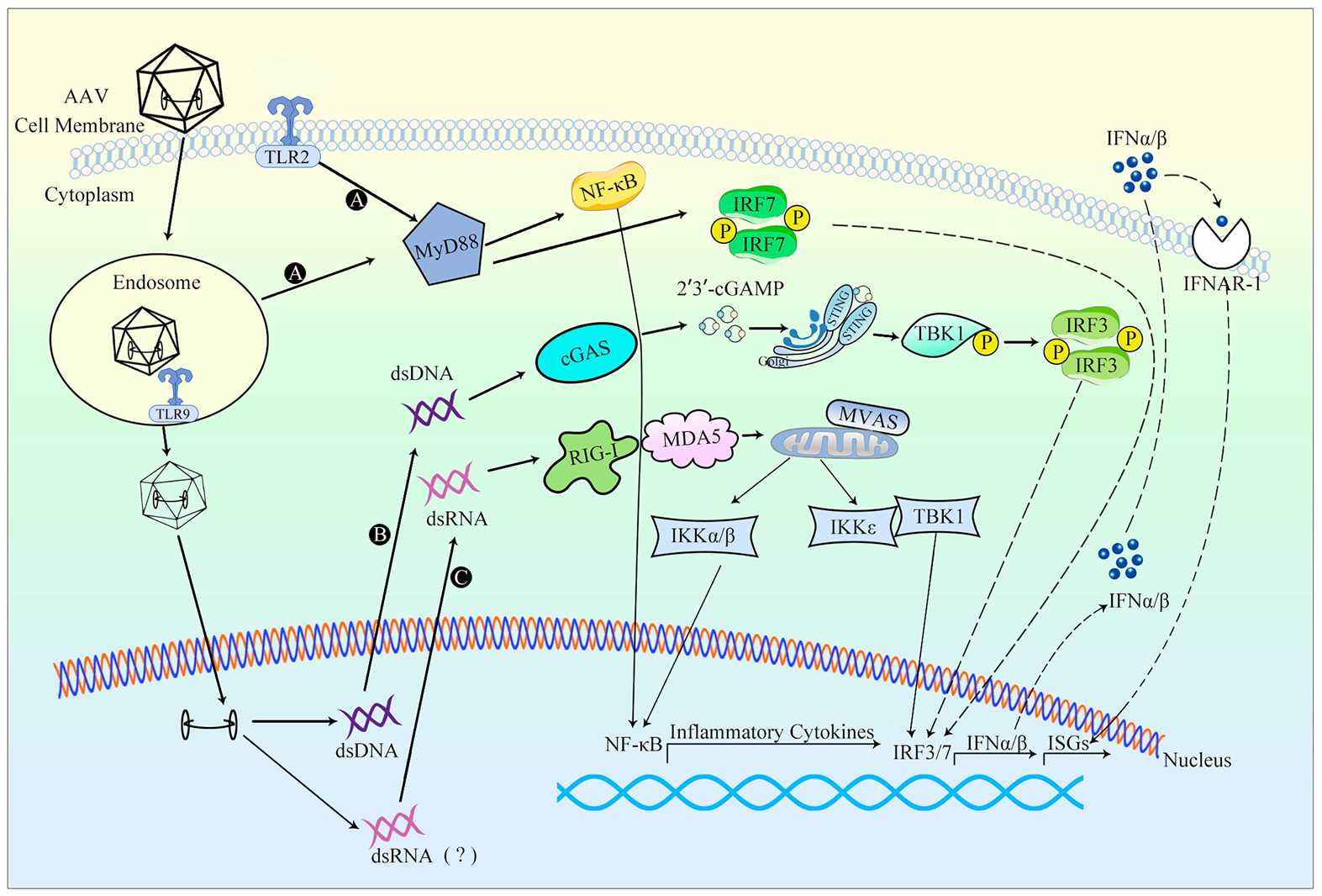 Fig.1 An overview of the innate response to AAV vectors 1,5.
Fig.1 An overview of the innate response to AAV vectors 1,5.
- Insertional Mutagenesis
The potential for insertional mutagenesis remains a major concern when using integrating viral vectors like lentivirus and retrovirus vectors. These vectors insert their genetic material into the host genome which may interfere with normal gene function or activate oncogenes. Retroviral vectors have extensive documentation of insertional mutagenesis risk because their integration into the host genome can activate nearby sequences which may result in oncogenesis.
Animal models in preclinical studies demonstrate tumor development when retroviral vectors activate oncogenes after their genetic material integrates into the host genome. A research study showed that mice gene therapy with retroviral vectors developed leukemia when the vector integrated near an oncogene. Due to their lower likelihood of causing insertional mutagenesis, lentivirus vectors are considered safer than retroviral vectors yet retain theoretical risks when used at high doses.
- Replication Competence
Replication-competent viral vectors create a major biosafety challenge due to their properties. Once inside a host, replication-competent viruses can spread throughout the body and infect other individuals which creates unexpected risks. The employment of replication-competent oncolytic viruses that target cancer cell replication generates concerns about environmental exposure risks.
Research conducted on replication-competent adenovirus vectors showed that these genetic vectors could remain in the environment and spread to unintended tissue areas. Strict biosafety protocols become essential when conducting research involving replication-competent vectors. Although lentivirus vectors originated from HIV are designed to be replication-incompetent, they must undergo careful assessment to confirm they do not recover replication abilities.
Strategies for Managing Safety Risks
- Vector Engineering for Safety
Self-inactivating (SIN) vectors have been engineered to improve safety measures. The design of these vectors excludes viral enhancer and promoter elements from the 3' long terminal repeat (LTR) which helps to lower insertional mutagenesis risks and reduces oncogene activation chances. SIN vectors play a critical role in lentivirus vector applications because they reduce the dangers of interfering with normal gene functions and generating unwanted genetic alterations through genomic integration.
The goal of targeted integration strategies is to guide viral vectors toward specific safe harbor genomic locations. Researchers utilize CRISPR/Cas9 technology to guide viral DNA integration into genomic regions that minimize disruption to crucial genes and activation of oncogenes. The approach increases lentivirus vector safety by reducing the potential for insertional mutagenesis.
Vectors can be modified to use immune-evasive promoters to reduce immune system responses. These promoters aim to reduce immune system activation while sustaining effective gene expression levels. The use of tissue-specific promoters enables the therapeutic gene to express only within desired cells which helps to minimize off-target effects and immune responses. MicroRNA target sequences enable control of gene expression and minimize off-target effects when incorporated into the system.
- Preclinical Testing for Safety
Immune response assays provide essential data to evaluate how viral vectors could trigger immune activation. The assays evaluate both cytokine production and immune cell activation following the delivery of vectors. AAV vector research indicates that specific serotypes produce minimal immune responses which makes them appropriate for applications that require low immune activation. The analysis of immune profiles of various vectors enables researchers to choose optimal vectors for their intended uses while reducing the possibility of negative immune responses.
The evaluation of safety and efficacy of custom viral vectors requires animal models including mice, rats and non-human primates in living organisms. These models reveal information about how therapeutic genes distribute throughout the body, how efficiently they can be converted into functional proteins, and how long these genes remain active. Researchers use rodent models to conduct preliminary safety and efficacy assessments but rely on large animal models to gather translational data needed to progress therapies toward clinical trials. Research with non-human primates established both the practicality and safety of applying AAV-mediated gene delivery to the retina which allowed these findings to advance into clinical trials targeting inherited retinal diseases.
- Monitoring and Control Strategies
The monitoring of safety and treatment success in gene therapy depends on analyzing biomarkers. Regular biomarker surveillance of cytokine levels, immune cell activation and gene expression levels enables early detection of negative immune responses or unintended effects during treatment. The presence of increased cytokine concentrations serves as an indicator of immune system activity and enables prompt treatment actions to handle possible negative outcomes.
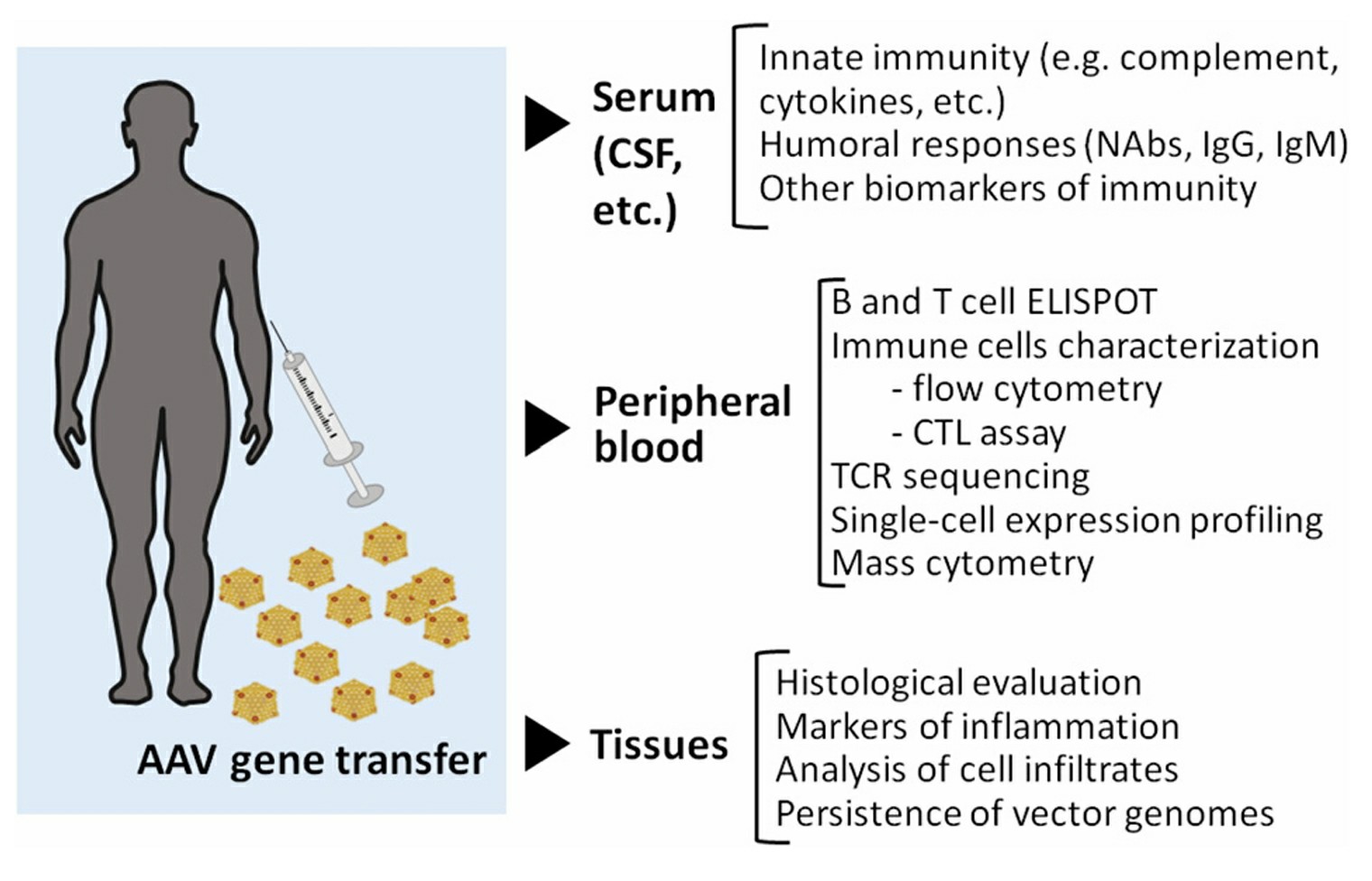 Fig.2 Immunomonitoring in gene transfer 2,5.
Fig.2 Immunomonitoring in gene transfer 2,5.
Researchers employ positron emission tomography (PET) and magnetic resonance imaging (MRI) imaging techniques to monitor the distribution and localization patterns of viral vectors throughout the body. The application of these techniques delivers important biodistribution data that confirms vector delivery to target tissues operates effectively. PET imaging enables visualization of therapeutic gene uptake and expression in target tissues which helps optimize vector delivery while confirming that therapy reaches its designated targets.
Case Studies of Safety Management
- AAV Vectors in Neurological Diseases
Preclinical studies have shown promising results. The use of AAV2-hAADC in rodent and non-human primate Parkinson's disease models led to substantial motor function enhancements and durable therapeutic gene expression. AAV5-miHTT treatment in Huntington's disease models produced substantial decreases in mutant huntingtin protein levels. Research demonstrates that AAV vectors function safely and effectively for neurological disease treatment while only causing minor side effects like neuroinflammation.
- Adenovirus Vectors in Cancer Therapy
Adenovirus vectors achieve high transduction efficiency combined with transient expression which qualifies them as appropriate tools for cancer therapy. Scientists have designed these vectors to transport tumor suppressor genes or prodrug-converting enzymes directly to cancer cells. The safety of adenovirus vectors in cancer therapy is enhanced through modifications that diminish immunogenicity and enhance targeting toward tumor cells.
Animal model research shows that adenovirus vectors effectively decrease tumor size while enhancing survival rates. The robust immune reaction generated by adenovirus vectors results in considerable inflammation and tissue injury. Researchers use less immunogenic serotypes and temporary immune suppression as approaches to reduce immune responses. Adenovirus vectors present potential for cancer treatment but need precise management of immune reactions to enhance their safety and effectiveness.
- Lentivirus Vectors in Hematopoietic Disorders
Researchers select lentivirus vectors for their ability to provide stable gene expression over long periods which is essential for hematopoietic stem cell transplantation procedures. The design of these vectors enables them to integrate into the host genome which maintains continuous therapeutic gene expression. The design of lentivirus vectors as SIN vectors enhances safety because these vectors exclude viral enhancer and promoter elements which helps to minimize insertional mutagenesis risks.
Research conducted in preclinical settings demonstrates that lentivirus vectors successfully transduce HSCs and provide enduring genetic defect corrections. Research models of severe combined immunodeficiency (SCID) and β-thalassemia showed substantial enhancement of immune function and hematopoietic parameters after using lentivirus vectors. Lentivirus vectors demonstrate significant promise as treatment methods for hematopoietic diseases because they maintain a safe profile through low occurrences of off-target effects and decreased chances of insertional mutagenesis.
Future Directions of Viral Vectors in Gene Therapy
Gene therapy research on viral vectors continues to advance through consistent innovation and refinement that improves their efficiency and safety while targeting specific genes. Current research aims to improve vector design by genetically modifying capsid proteins to avoid immune detection and implementing tissue-specific promoters for precise gene expression. Targeted integration strategies using CRISPR/Cas9 technology with SIN vectors represent essential research to lower insertional mutagenesis risks while minimizing off-target effects. AI and machine learning technologies will transform vector design by enabling precise targeting capabilities and personalized therapeutic approaches. Researchers are studying new delivery techniques including advanced nanoparticles and lipid-based carriers to enhance vector stability while minimizing immunogenic responses. Research through both preclinical and clinical studies reveals essential information about vector safety and effectiveness which guides the development of better therapies for numerous genetic and acquired diseases. The progression of gene therapy research could revolutionize medical treatments by increasing their accessibility and enhancing patient results.
References
- Wang, Y.; Shao, W. Innate Immune Response to Viral Vectors in Gene Therapy. Viruses. 2023, 15, 1801. https://doi.org/10.3390/v15091801.
- Ronzitti, G.; Gross, D-A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11:670. https://doi.org/10.3389/fimmu.2020.00670.
- Ma, W.; Wu, Z.; Zhao, T. Preclinical evaluation of AAV9-coSMN1 gene therapy for spinal muscular atrophy: efficacy and safety in mouse models and non-human primates. Mol Med. 2025, 31, 158. https://doi.org/10.1186/s10020-025-01207-4.
- Alsalloum, A.; Gornostal, E.; Mingaleva, N.; Pavlov, R.; Kuznetsova, E.; Antonova, E.; Nadzhafova, A.; Kolotova, D.; Kadyshev, V.; Mityaeva, O.; et al. A Comparative Analysis of Models for AAV-Mediated Gene Therapy for Inherited Retinal Diseases. Cells. 2024, 13, 1706. https://doi.org/10.3390/cells13201706.
- Distributed under Open Access license CC BY 4.0, without modification.
