The Immune Response Conundrum: Viral Vectors and Preclinical Gene Therapy
Viral vectors for gene therapy
- AAV vector gene therapy
The most common viral vector for in vivo gene therapy treatments comes from Adeno-associated virus (AAV). The parvovirus AAV contains a 4.7 kilobase pair DNA genome within a nonenveloped icosahedral protein shell and does not cause disease. There are 11 natural serotypes of AAV and it has more than 100 different variants. Each AAV serotype demonstrates specific tissue tropism which enables them to deliver genes to particular tissue types. AAV9 targets central nervous system organs through its tropism while AAV8 effectively delivers transduction to pancreatic tissues. Major characteristics of AAV vectors include: AAV vectors transduce both dividing and non-dividing cells without integrating DNA into the host genome and they allow long-term stable gene expression with low immunogenicity. The distinct properties of AAV make it the ideal choice for in vivo gene therapies to treat conditions needing lasting gene modifications.
- Adenovirus vector gene therapy
The clinical investigation for in vivo gene therapies began with the use of Adenovirus (Ad) as one of the earliest viral vectors. Ads represent a group of DNA viruses that contain a 34–43 kb genome within a nonenveloped icosahedral viral particle. The Adenovirus group contains over 50 serotypes but Ad5 and Ad26 remain the preferred choices for gene therapy applications. Scientists have designed three generations of Ad vectors to serve various therapeutic uses. The initial generation of Ad vectors becomes capable of transporting up to 7.5 kb foreign DNA after removal of the E1 and E3 units. The elimination of E1 and E4 units in second-generation Ad vectors results in substantial immunogenicity reduction. The third-generation vector contains no viral genes which enables it to carry foreign DNA sequences longer than 30 kb. Notable characteristics of Ad vectors include: The delivery of multiple genome copies into a single host cell leads to elevated gene expression levels through Ad vectors. The particular properties of Ad vectors drive their main application in cancer therapy and infectious disease vaccination.
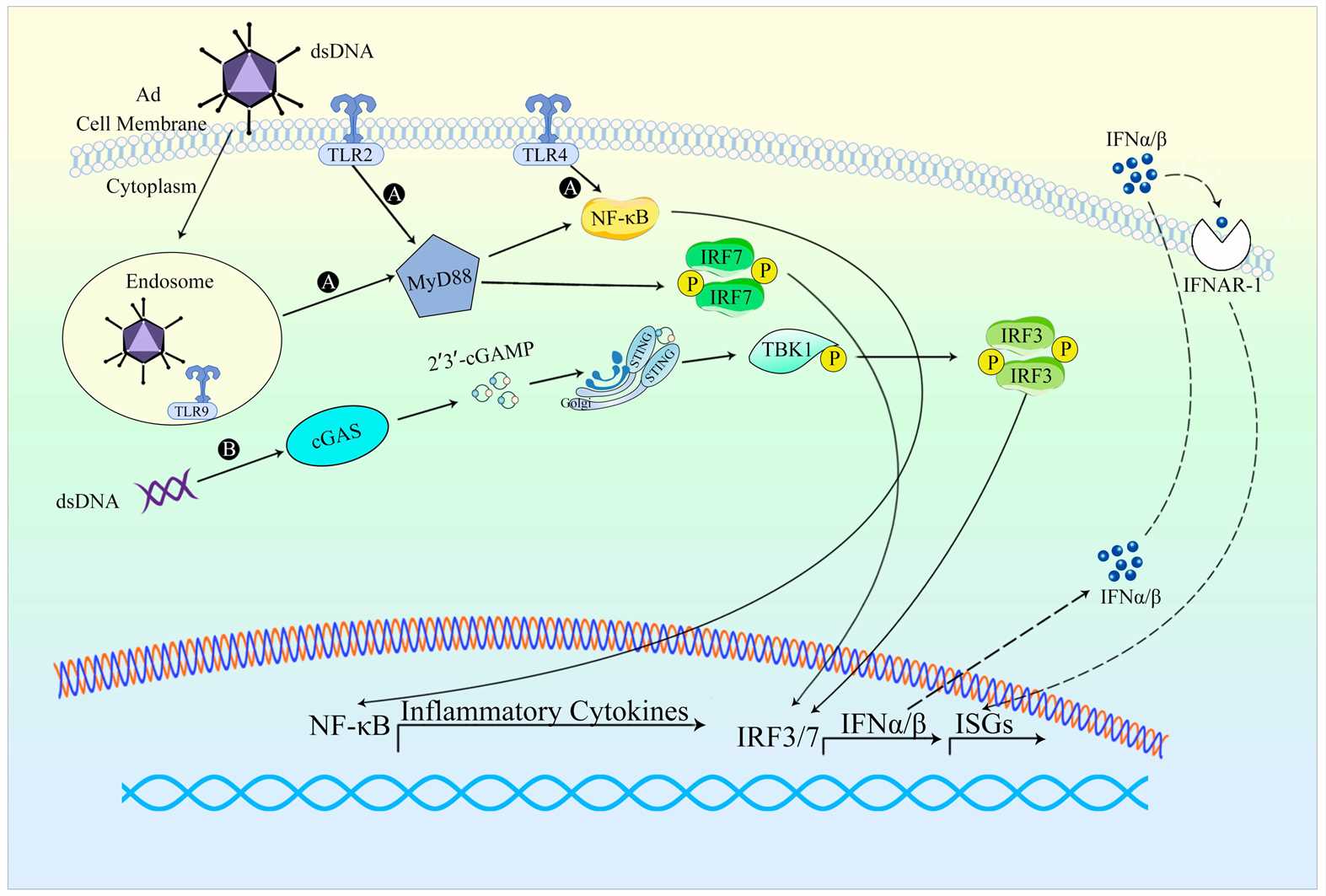 Fig.1 Genome structure description of Ad, AAV, and LV viruses and their viral vectors1,6.
Fig.1 Genome structure description of Ad, AAV, and LV viruses and their viral vectors1,6.
- Lentiviral vector gene therapy
Lentivirus stands as a crucial viral vector employed in gene therapy techniques. The primary use of this system involves ex vivo gene therapy applications. Clinical investigations are currently underway to explore its applications for in vivo gene therapy. The lentivirus represents a specific type of retrovirus which transports genetic information through RNA strands. The ability of lentivirus to integrate its genome into nondividing cells distinguishes it from retroviruses which lack this capability. Lentivirus vectors from the first generation originated from HIV-1 and demonstrated their ability to transduce brain organs in living organisms. The latest lentivirus vectors come from nonhuman lentiviruses which are theoretically safer because their original viruses cannot infect humans. Distinguishing characteristics of lentivirus vectors include: Lentivirus vectors can deliver genes to both dividing and nondividing cells while maintaining long-term gene expression and showing less genotoxicity and insertional mutagenesis risk than retrovirus vectors. The primary limitation of lentivirus vectors lies in their restricted capacity to carry genetic material.
- Herpes simplex virus (HSV) vector gene therapy
HSV contains an envelope structure and possesses a genome composed of double-stranded NDA nucleic acid which extends beyond 150 kb in length. The Herpes simplex virus genome contains about 90 genes where half are nonessential allowing removal/replacement in recombinant vectors to achieve high foreign DNA carrying capacity. Research has discovered eight different human HSV serotypes which demonstrate unique tissue preferences. Three main HSV vector types engineered for gene therapy purposes exist which comprise amplicon HSV, replication-defective HSV, and replication-competent HSV. Amplicon HSV functions as an engineered vector that can transport foreign DNA exceeding 100 kilobases. The replication-defective HSV vector becomes non-pathogenic through the removal of genes essential for initiating the HSV lytic cycle. The replication-competent HSV vector represents an engineered HSV that maintains its replication genes for use in vitro while lacking the replication genes in vivo. Major characteristics of HSV vectors include: HSV vectors possess three main features which are immune system evasion capabilities along with the ability to deliver large DNA payloads containing multiple genes and having intrinsic or engineered abilities to target specific cells with lytic activity. The primary focus of HSV vector-based gene therapies in clinical practice has been cancer treatment because these vectors possess innate oncolytic properties.
- Retrovirus vector gene therapy
Retrovirus served as the initial viral vector examined in clinical trials for in vivo gene therapy applications. Retroviruses present as enveloped spherical viruses which transport their genetic information through RNA. Retrovirus vectors convert their single-stranded RNA genetic materials into double-stranded DNA before integration into the host cell genome. Retrovirus vectors stand out in gene therapy because they possess the ability to carry large genes between 9–12 kb which enables them to integrate into host genomes for prolonged gene expression. Retrovirus vectors need actively dividing cells to perform genomic integration which limits their transduction ability to such cells only. If DNA from retrovirus vectors inserts itself randomly into the host chromosome it can cause insertional mutagenesis. Scientists created self-inactivating vectors which lack the promotor or enhancer of the long terminal repeat to lower insertional mutagenesis risks. Retrovirus vectors have become less common in clinical studies because of their limitations.
Types of Immune Responses
- Innate Immune Response
When pathogens invade the body, the innate immune response serves as the primary defense system to protect against infection. The innate immune response offers non-specific protection against infections by serving as a universal defensive barrier. The skin functions as a physical barrier that prevents pathogens from entering the body. The mucous membranes of the respiratory system along with those of the digestive system and urogenital tract function to capture and eliminate microbial invaders. Stomach acid enzymes destroy ingested pathogens while saliva and tears fight bacteria through lysozymes that break down their cell walls. Macrophages and neutrophils function as phagocytes because they engulf pathogens and then break them down. Natural Killer cells destroy cells that have been infected by viruses as well as tumor cells. Dendritic cells function as antigen-presenting cells (APCs) that trigger adaptive immune responses. Cytokines and chemokines serve as signaling molecules that guide immune cells toward infection sites thus producing redness and swelling with added heat and pain. A collection of proteins helps destroy pathogens by both enhancing phagocytosis and creating pores in microbial membranes.
- Adaptive Immune Response
The adaptive immune response represents a specialized system that specifically attacks particular pathogens. This immune response needs previous contact with an antigen to function and retains memory which leads to lasting protection. The primary elements of the adaptive immune response consist of:
B cells generate antibodies that attack pathogens directly or tag them for immune system destruction. The main antibody types consist of IgG, IgA, IgM, IgE and IgD. Plasma cells generate large quantities of antibodies and memory B cells store pathogen data to enable quicker future immune responses. CD4+ Helper T cells stimulate both B cells and other immune cells to function properly. Cytotoxic T cells (CD8+) eliminate cells that are infected with viruses as well as cancerous cells. Regulatory T cells preserve immune balance while blocking the development of autoimmune diseases. Memory T cells create quicker immune reactions when exposed to familiar pathogens.
Specific Immune Reactions to Different Viral Vectors
AAV Vectors in Gene Therapy
- Pre-existing Immunity and Its Impact on Transduction
AAV vectors exhibit low immunogenicity yet their effectiveness can be greatly reduced by existing immune responses. If antibodies against AAV capsids exist in the patient's body they will neutralize the vector which blocks its entry into target cells thereby decreasing transduction efficiency. Different serotypes of AAV show varying levels of pre-existing immunity depending on geographical location as studies demonstrate seropositivity rates between less than 10% and more than 90%. The presence of pre-existing immunity remains a significant obstacle for systemic AAV administration because low levels of neutralizing antibodies (NAbs) can substantially diminish transgene expression.
- Case Studies Demonstrating Immune Responses to AAV
An AAV-based gene therapy clinical trial for severe hemophilia B showed that two out of seven patients developed a cytotoxic T lymphocyte (CTL) response against the AAV capsid which became apparent through temporary liver enzyme level increases. Animal models failed to predict this response yet researchers have faced difficulties reproducing it in laboratory settings. A separate investigation showed that existing NAbs with high titers against the AAV2 capsid reduced transduction efficiency in mice. The results emphasize that evaluating pre-existing immunity in patients is crucial before administering AAV-based gene therapy.
Adenovirus Vectors in Gene Therapy
- Strong Immune Response and Inflammation
The innate immune system primarily generates strong immune reactions when exposed to adenovirus vectors. Substantial inflammation occurs when high doses of adenovirus vectors are administered owing to the production of pro-inflammatory cytokines and chemokines. When immune responses become active they harm tissue and reduce the effectiveness of transduction. Adenovirus vectors lead to powerful adaptive immune responses by activating memory T cells.
- Case Studies Demonstrating Immune Responses to Adenovirus
Ad5 vector systems generated greater gene expression levels and produced stronger immune responses compared to other adenovirus serotypes during the development of HIV vaccines. The powerful immune reaction caused functional exhaustion and reduced vaccine performance after booster shots were given. Studies revealed that simultaneous use of Ad26 and Ad5 adenovirus serotypes for expressing the Simian Immunodeficiency Virus (SIV) Gag protein improved both cell-mediated immune responses and survival rates in monkeys exposed to SIV. Scientific studies demonstrate the importance of balancing the immune response generated by adenovirus vectors with their capacity to deliver genetic material.
Lentivirus Vectors in Gene Therapy
- Integration and Potential for Long-term Immune Response
Lentivirus vectors originate from retroviruses and insert their DNA into the host genome which achieves stable gene expression over an extended period. The integration of viral vectors causes extended immune responses because cytotoxic T cells (CD8+ T cells) attack the modified cells. The process of integrating lentivirus vectors activates innate immune responses including type I interferons (IFNα/β) production which then enhances inflammation.
- Case Studies Demonstrating Immune Responses to Lentivirus
Research demonstrated that intravenous delivery of VSV-pseudotyped lentivirus vectors in mice for gene therapy led to increased IFNα/β levels in the liver and spleen with subsequent reductions in proviral DNA content. The clearance of the vector from the body required the innate immune response mechanism. Research has shown that lentivirus vectors using tissue-specific promoters decrease unintended expression and immune system activation. The research underscores the necessity of tailoring vector design so that it reduces immune reactions yet preserves its therapeutic effectiveness.
Impact on Gene Therapy Efficacy and Safety
- Reduction in Transduction Efficiency Due to Immune Clearance
The efficiency of viral vector transduction during gene therapy treatment may decrease when immune responses interfere with the process. The immune system eliminates vector particles and kills transduced cells which leads to this outcome.
The presence of pre-existing immunity along with quick immune system activation results in viral vector neutralization and removal before they can deliver genetic material to target cells. Pre-existing neutralizing antibodies attach to the AAV vector capsid which blocks its entry into target cells. The potent immunogenic nature of adenovirus vectors leads to their quick clearance by the immune system which prevents many vectors from reaching their target tissue.
Cells that have undergone transduction by viral vectors become targets for destruction by the immune system. The integration of lentivirus genetic material into host genomes poses a problem because it can lead to cells being targeted for destruction by the immune system. CD8+ T cells function to identify and destroy cells that have received transduction which results in decreased numbers of cells expressing the therapeutic gene.
- Inflammation and Potential for Adverse Effects
The immune system's reaction to viral vectors produces inflammation and various negative effects which threaten both the safety and effectiveness of gene therapy treatments. When the innate immune response becomes active at the vector administration site it results in inflammation. Adenovirus vectors frequently trigger the generation of pro-inflammatory cytokines and chemokines. Inflammatory responses result in tissue damage and reduced gene delivery efficiency while also causing systemic adverse effects. Several preclinical and clinical studies have shown that administering high adenovirus vector doses results in severe liver toxicity alongside systemic inflammatory responses.
The immune system's reaction to viral vectors causes several side effects from fever and fatigue up to severe organ toxicity. These adverse effects have the potential to become life-threatening in certain situations. During a clinical trial for adenovirus vector-based gene therapy one patient experienced severe systemic inflammatory response syndrome (SIRS) which resulted in multiple organ failure and death. Effective management of immune responses emerges as an essential requirement to maintain gene therapy safety.
Case Studies: Immune Response Studies in Preclinical Settings
- AAV vector gene therapy in retinal disease models
Retinal gene therapy preclinical models have employed AAV vectors like AAV2, AAV5, and AAV8 extensively. Retinal cells are efficiently transduced by these vectors because of their low immunogenic profiles. Even though these vectors demonstrate low immunogenicity, higher doses or multiple administrations can trigger immune responses. The dual AAV8 vectors delivered large genes such as MYO7A in mouse models which resulted in substantial improvements in retinal structure and function when administered at high doses. The body's immune response to AAV vectors can be assessed by tracking both neutralizing antibodies (NAbs) and T-cell responses. Researchers conduct preclinical tests with mouse models alongside human retinal explants (HREs) and retinal organoids from induced pluripotent stem cells (iPSCs) to evaluate AAV-mediated gene therapy effectiveness and safety.
- Adenovirus vector gene therapy in cancer models
Adenovirus vectors deliver genes efficiently but provoke strong immune responses that cause inflammation and harmful effects. Scientists developed modified adenovirus vectors using alterations to fiber proteins and tissue-specific promoters to address these challenges. Researchers have developed adenovirus vectors to express tumor suppressor genes within cancer cells which helps to minimize off-target effects in cancer models. Preclinical immune response evaluations require measurements of cytokine levels as well as activation of T-cells and the detection of neutralizing antibodies. These studies demonstrate how therapeutic effectiveness and immune-mediated toxicity can be managed together.
- Lentivirus vectors in hematopoietic disorders
Lentivirus vectors integrate into the host genome to deliver stable long-term gene expression. Engineered envelope proteins and tissue-specific promoters in these vectors improve transduction efficacy while minimizing immune reactions. In HSC transplantation models researchers have developed lentivirus vectors that deliver functional genes to repair genetic defects in HSCs. Researchers assess immune responses in these models by tracking cytotoxic T cell activation (CD8+ T cells) and measuring NAb levels. Mouse models serve as the primary platform for preclinical studies that evaluate both safety and effectiveness of gene therapy delivered through lentiviruses.
Strategies to Modulate Immune Responses
- Vector Modifications to Reduce Immunogenicity
AAV Vectors: Altering AAV vector capsid proteins decreases immune system recognition. Reducing immunogenicity by selecting less immunogenic AAV serotypes or altering the capsid to prevent neutralizing antibody binding can improve transduction efficiency. The creation of AAV variants with diminished immunogenicity has been achieved through the application of directed evolution and rational design techniques.
Adenovirus Vectors: The immunogenicity of adenovirus vectors decreases through modifications made to their fiber proteins. Customizing the fiber knob domain to bind specific cell surface receptors enables better cellular entry alongside diminished immune detection.
Lentivirus Vectors: By altering lentivirus vector envelope proteins with VSV-G from vesicular stomatitis virus researchers can improve transduction efficiency while also minimizing immune responses. Researchers are actively studying how to modify envelope proteins so they avoid detection by the immune system.
- Use of Immune-Evasive Promoters and Regulatory Elements
AAV Vectors: The use of tissue-specific promoters ensures that therapeutic gene expression occurs exclusively within target tissues to minimize side effects and immune reactions. Neuron-specific expression in the CNS utilizes the synapsin I promoter while the muscle creatine kinase (MCK) promoter achieves muscle-specific expression.
Adenovirus Vectors: The use of tissue-specific promoters within adenovirus vectors results in improved targeted gene expression while decreasing immune responses. The albumin promoter allows for liver-specific expression that minimizes unintended effects in other tissues.
Lentivirus Vectors: Lentivirus vectors require tissue-specific promoters to achieve targeted gene expression. The CD34 promoter in lentivirus vectors provides selective expression in hematopoietic stem cells (HSCs).
- Future Innovations in Immune Response Management
Synthetic Biology Approaches: Synthetic biology enables the development of new vectors which possess both lower immunogenicity and improved targeting functions. Researchers can develop vectors with combined capsid proteins and add synthetic regulatory elements to achieve desired outcomes.
CRISPR/Cas9 Integration: Precise gene editing is achieved by combining CRISPR/Cas9 technology with viral vectors which also work to reduce immune responses. Researchers can deploy CRISPR/Cas9 technology to disable immune-activating genes in either the vector or host cells.
Immunosuppressive Agents: Targeted immunosuppressive treatments enable management of specific immune responses while maintaining general immune system function. Researchers can specifically block immune activation through the application of small molecules alongside monoclonal antibodies and other biologic agents.
Immune Tolerance Induction: We need to create methods that will help the immune system accept therapeutic gene products without attacking them. The process may include antigen-specific tolerance induction methods which encapsulate the therapeutic gene product in nanoparticles or utilize tolerogenic dendritic cells.
Comparative Analysis
- Comparison of Immune Responses Across Vector Types
AAV vectors activate the innate immune system through TLR2 and TLR9 pathways which results in mild inflammation. The adaptive immune response which includes CD8+ T cells and neutralizing antibodies (NAbs) production limits gene therapy effects. Adenovirus vectors activate a strong innate immune response which leads to substantial inflammation through pro-inflammatory cytokines and chemokines activation. These vectors induce a powerful adaptive immune response which activates CD4+ and CD8+ T cells and generates high levels of neutralizing antibodies leading to swift vector elimination. Lentivirus vectors create only moderate innate immune responses and activate CD4+ T cells and NAbs but these responses remain weaker compared to the reactions elicited by adenovirus vectors. AAV vectors induce minimal immune response which makes them optimal for use in medical procedures that require minimal immunogenic activity.
- Impact on Long-Term Expression and Stability
The immune response of each vector type determines the stability and expression duration of delivered therapeutic genes. AAV vectors deliver stable expression over extended periods which proves vital for chronic disease treatment. Pre-existing immunity along with NAbs development reduces AAV-based therapy efficacy and hinders subsequent treatments. Adenovirus vectors usually provide temporary gene expression that limits their use in prolonged treatments. The intense immune response triggered by these vectors results in transduced cell elimination which shortens gene expression timeframes. The ability of lentivirus vectors to integrate their genetic material into the host genome results in stable long-term gene expression. Ensuring stable and safe gene expression requires careful management of the risks associated with insertional mutagenesis along with the potential for long-term immune responses.
- Suitability for Different Types of Gene Therapy Applications
Gene therapy applications determine which vector is used because each application has unique requirements. AAV vectors provide stable long-term gene expression in post-mitotic cells like neurons and muscle cells which makes them appropriate for neurodegenerative disease and muscular dystrophy treatment. These applications benefit significantly from their reduced immunogenic properties. Adenovirus vectors make excellent choices for applications which need to achieve high-level transient gene expression including cancer gene therapy and vaccine development. The capacity to transport extensive genes and induce powerful immune responses proves advantageous in these situations. Lentivirus vectors enable stable gene expression over long periods in both dividing and non-dividing cells which is why they are used in hematopoietic stem cell transplantation and chronic genetic disease treatment. Genomic integration capability allows these systems to maintain gene expression yet requires the implementation of meticulous vector design and safety evaluations to manage potential risks.
References
- Wang, Y.; Shao, W. Innate Immune Response to Viral Vectors in Gene Therapy. Viruses. 2023, 15, 1801. https://doi.org/10.3390/v15091801.
- Zhao, Z.; Anselmo, AC.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng Transl Med. 2022, 7(1):e10258. https://doi.org/10.1002/btm2.10258.
- Wang, JH., Gessler, D.J., Zhan, W. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Sig Transduct Target Ther. 2024, 9, 78. https://doi.org/10.1038/s41392-024-01780-w.
- Araújo, N.M.; Rubio, I.G.S.; Toneto, N.P.A. The use of adenoviral vectors in gene therapy and vaccine approaches. Genet. Mol. Biol. 2022, 45: e20220079. https://doi.org/10.1590/1678-4685-GMB-2022-0079.
- Alsalloum, A.; Gornostal, E.; Mingaleva, N.; Pavlov, R.; Kuznetsova, E.; Antonova, E.; Nadzhafova, A.; Kolotova, D.; Kadyshev, V.; Mityaeva, O.; et al. A Comparative Analysis of Models for AAV-Mediated Gene Therapy for Inherited Retinal Diseases. Cells. 2024, 13, 1706. https://doi.org/10.3390/cells13201706.
- Distributed under Open Access license CC BY 4.0, without modification.
