Navigating the Viral Vector Landscape: A Comprehensive Review of AAV, Adenovirus, and Lentivirus in Preclinical Gene Therapy
Vectors in gene therapy
- Non-Viral Vectors for gene therapy
Researchers have prioritized the development of non-viral vectors in gene therapy to overcome the high costs and complex production processes of viral vectors and their ability to trigger inflammatory responses. Non-viral vectors provide an inexpensive and straightforward yet efficient solution for gene therapy. Many non-viral vectors have been developed which exhibit distinct benefits and drawbacks. Lipid nanoparticles (LNPs) represent a non-viral vector category which emerges from the arrangement of phospholipid biomolecules. Through their membrane fusion ability these biomolecules simultaneously carry water-soluble and fat-soluble drugs and deliver genetic drugs to body cells. LNPs can only carry about 22 base pairs of nucleic acid sequences yet provide multiple benefits including biocompatibility and reduced toxic effects while helping to overcome drug resistance and enabling endosomal escape. The advantageous properties of LNPs make them a preferred choice for gene therapy treatments.
- Viral Vectors
Viruses act as nature's primitive gene therapy agents because they have evolved mechanisms to enter cells and distribute their genetic material efficiently. Researchers have repurposed viruses for human gene therapy by substituting their natural genome with therapeutic genes. Scientists' modification of viruses for therapeutic purposes resulted in the creation of multiple viral vectors currently used in gene therapy.
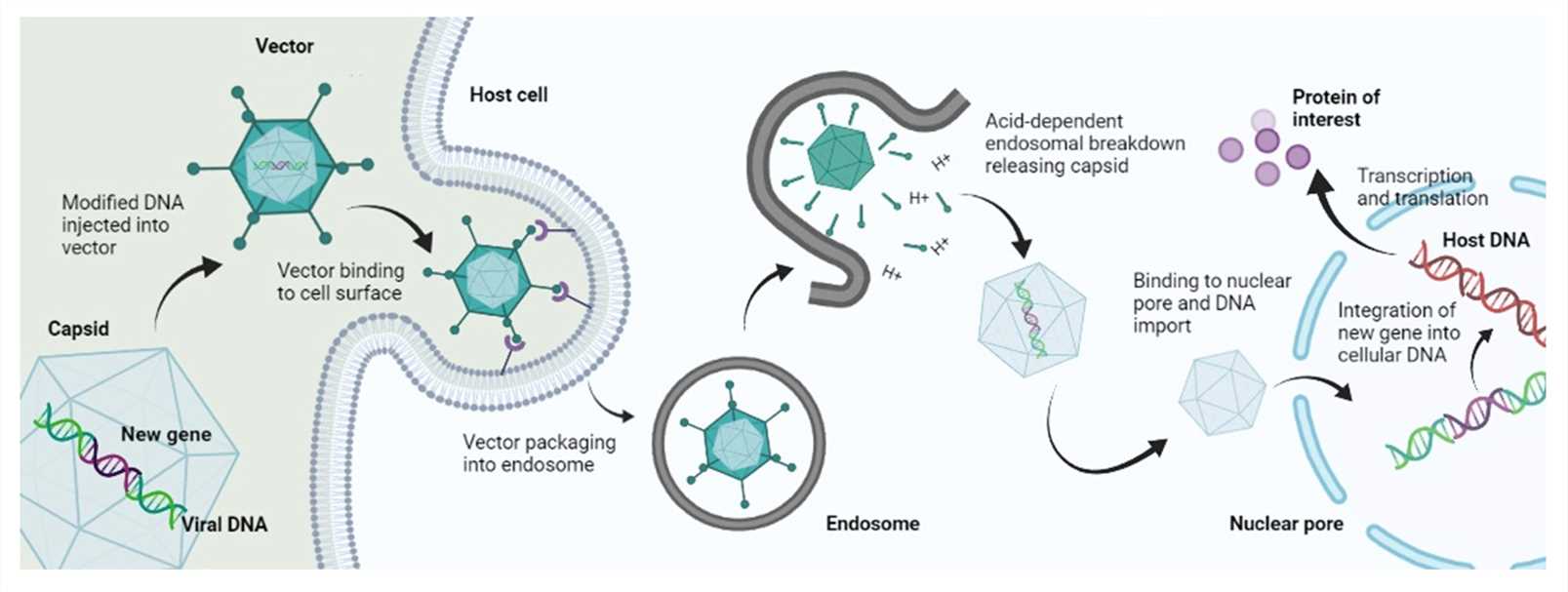 Fig. 1 Mechanism of viral gene delivery1,6.
Fig. 1 Mechanism of viral gene delivery1,6.
- Retroviral Viral Vectors
Ex vivo gene therapy involves the genetic modification of cells outside the body before reintroducing them to the patient. Clinical trials have utilized retroviral vectors since they represent a standard choice for viral vectors in ex vivo gene therapy applications. Retroviruses serve as RNA viruses which replicate through a DNA intermediate stage. The vectors enable scientists to performex vivo gene therapy for modifying CD34+ bone marrow hematopoietic stem cells and peripheral blood lymphocytes. The retroviral vector is unable to transport genetic material larger than 10 kb due to its 10 kb size limit.
- Lentiviral Vector (LVV)
LVVs represent a viral vector type which features a spherical form and contains single-stranded RNA. Ex vivo gene therapy applications for T cells frequently utilize these vectors. LVVs support larger genes because they accommodate genetic material up to 8 kb in size. LVVs stand out because they can accomplish continuous gene transfer within cells that divide. Safety concerns exist with these vectors because they might create unwanted mutations at non-target sites.
- Adeno-Associated Virus (AAV)
The non-enveloped double-stranded DNA virus known as AAV remains a popular choice for gene therapy applications because of its small size. AAV demonstrates excellent biological properties and lacks immunogenicity and pathogenicity which makes it an ideal option forin vivo gene therapy. The high gene transduction efficiency and large-scale ease of use of AAV have led to its application in numerous somatic gene therapy techniques.
- Adenovirus (AdV)
AdV functions as an in vivo somatic viral vector featuring an icosahedral nucleocapsid structure. The purification process for this substance is straightforward and it maintains genetic stability while supporting foreign gene loading up to 48 kb which ensures effective transduction across diverse tissues.
- Herpes Simplex Virus Type 1 (HSV-1)
HSV-1 stands out as an effective viral vector for delivering somatic gene therapy treatments directly within living organisms. HSV-1's ability to package large transgene cassettes with a capacity of up to 40 kb in replication defective form and 150 kb in amplicon form establishes it as a favorable choice for gene therapy. HSV-1 has a strong preference for neurons which makes it an excellent therapy choice for central nervous system diseases.
- Alphavirus
Alphaviruses with single-stranded RNA genomes serve as viral vectors for delivering genes to somatic cells within living organisms through gene therapy applications. The structure of these alphavirus particles includes a protein capsid which is encased by spike membrane proteins. Alphavirus particles bind to mammalian and insect cell surface proteins including laminin and heparin receptors which enables delivery of the RNA genome to the cytoplasm for direct RNA replication.
Adeno-associated virus vector as a platform for gene therapy delivery
Structure and characteristics of AAV vectors
AAVs are tiny viruses that lack an envelope with an icosahedral shape and measure approximately 25 nm across. The AAV genome includes a single-stranded DNA molecule that measures approximately 4.7 kb and features inverted terminal repeats (ITRs) on both ends. The AAV genome contains two main open reading frames (ORFs): The rep gene produces the proteins necessary for viral replication while the cap gene produces the capsid proteins VP1, VP2, and VP3. The viral shell that enables infection of host cells is formed by capsid proteins. AAVs need to infect host cells together with helper viruses like adenovirus or herpes simplex virus to finish their replication process. The therapeutic gene replaces viral genes in AAV vectors and gets flanked by ITRs during vector engineering. The vector design enables delivery and expression of the therapeutic gene within target cells. The tissue-specific binding sites present on the AAV capsid define its tropism which researchers can use to precisely target particular tissues during gene therapy.
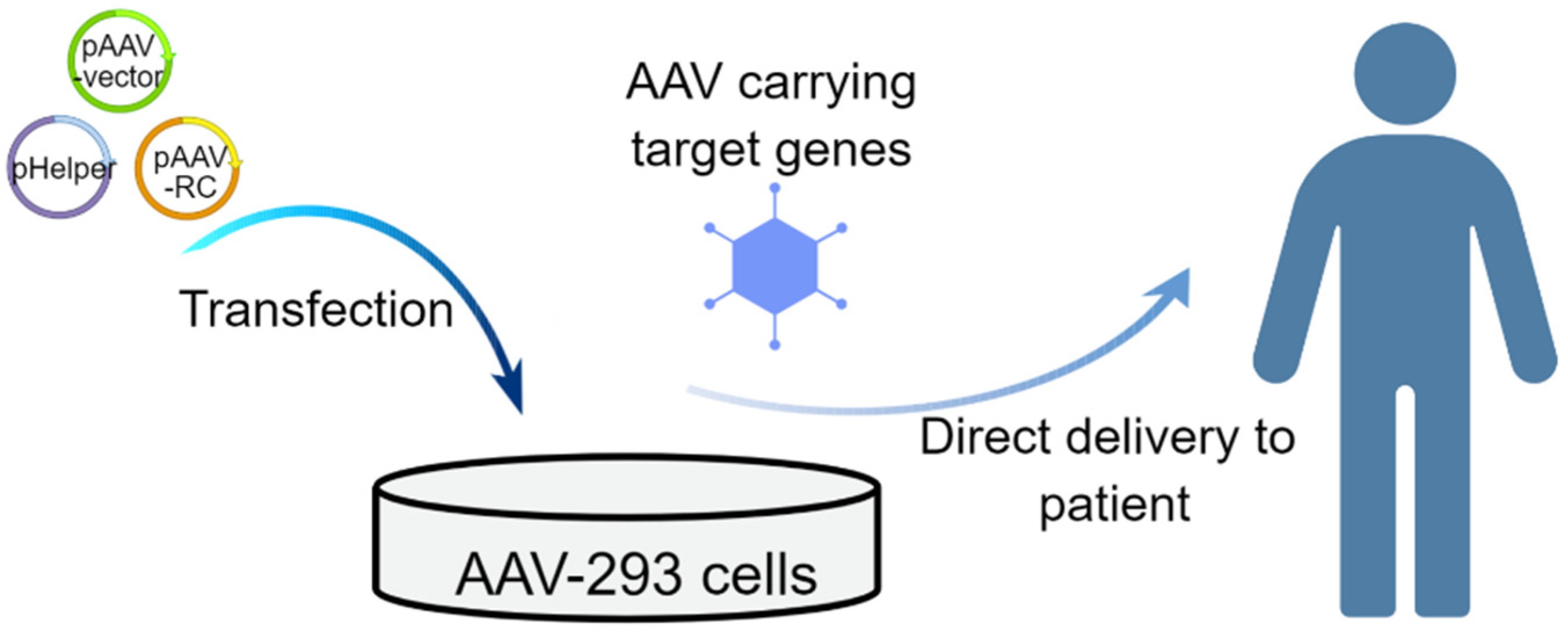 Fig.2 Schematic of the in vivo strategies that use AAV vectors for treating genetic diseases2,6.
Fig.2 Schematic of the in vivo strategies that use AAV vectors for treating genetic diseases2,6.
Advantages in Preclinical Studies
- Low Immunogenicity
The minimal immunogenicity of AAV vectors provides a major benefit for gene therapy applications. AAVs generally cause minimal immune responses which permits multiple treatments without increasing adverse reaction risks unlike adenoviral vectors. The ability to administer multiple doses without strong immune response makes this feature essential for long-term treatments and targeting tissues needing repeated dosing.
- Broad Tropism
AAV vectors have the capacity to infect numerous cell types including both dividing and non-dividing cells which allows these vectors to target multiple tissues and organs. The wide range of target tissues achieved by AAV vectors results from having 13 different serotypes each with unique receptor-binding characteristics suitable for directed tissue targeting. AAV serotype 2 (AAV2) targets neurons with high specificity while AAV serotype 8 (AAV8) demonstrates efficient hepatocyte transduction.
- Long-term Gene Expression
AAV vectors enable the stable expression of therapeutic genes over extended periods. The therapeutic gene remains active for many years because viral DNA either integrates into the host genome at a specific location or exists as an episome. Long-term correction of genetic defects is essential for treating chronic genetic diseases which makes this approach extremely useful.
Challenges and Limitations
- Limited Packaging Capacity
The main drawback of AAV vectors is their limited ability to package genetic material. The AAV genome can accommodate therapeutic genes up to 4.7 kb in length as its maximum size. The maximum payload size of AAV vectors restricts the delivery of large genes and poses difficulties for simultaneous delivery of multiple genes.
- Pre-existing Immunity
The existence of pre-existing immunity against AAV represents a major obstacle for successful gene therapy implementation. Natural infections with wild-type AAV have resulted in many people developing neutralizing antibodies which compromise the success of AAV-based gene therapy methods. Scientists can address pre-existing immunity to AAV through two main strategies which involve selecting non-immunogenic AAV serotypes and applying immune-evading techniques such as temporary immune suppression.
- Manufacturing Complexity
AAV vector production demands significant technical and financial investment. The production procedure begins with the co-transfection of producer cells using plasmids that carry both the AAV genome and essential helper functions before extracting the purified viral particles. Clinical applications depend on producing high-quality and high-yield AAV vectors which necessitate specialized facilities and expert knowledge.
Case Studies: Applications in Cell and Animal Models
- Treatment of Leber Congenital Amaurosis (LCA)
Leber congenital amaurosis represents a serious genetic condition which leads to blindness caused by RPE65 gene mutations. Preclinical studies utilized AAV vectors to insert functional RPE65 genes into retinal cells within animal models. Research data revealed major enhancements in visual abilities as treated animals experienced restored retinal function together with better light-sensing performance.
- Duchenne Muscular Dystrophy (DMD)
The dystrophin gene mutations lead to Duchenne muscular dystrophy which results in progressive muscle degeneration. Preclinical studies have utilized AAV vectors to transport micro-dystrophin genes into muscle cells of animal models. Research has demonstrated that delivering micro-dystrophin genes through AAV vectors produces functional micro-dystrophin proteins which enhance muscle function while decreasing muscle degeneration. Researchers are currently conducting human clinical trials to evaluate AAV-based gene therapy as a treatment option for DMD.
- Hemophilia B
Hemophilia B represents a genetic condition stemming from an insufficient amount of clotting factor IX. Researchers demonstrated that AAV vectors delivering the factor IX gene to liver cells in animal models produced long-term functional expression of factor IX which resulted in better blood clotting and fewer bleeding episodes.
Adenovirus Vectors in Gene Therapy
- Structure and characteristics of adenovirus vectors
Adenovirus-derived AdVs originate from non-enveloped double-stranded DNA viruses with genome sizes between 36 and 40 kilobase pairs. The detailed characterization of the adenovirus genome enables simple manipulation which produces both replication-defective and replication-competent recombinant vectors. The viral capsid structure consists of fiber proteins, penton base and hexon proteins that enable cell attachment and entry. The AdV system supports the transfer of substantial transgenes matching their genome size while achieving high transduction success rates in both dividing and non-dividing cellular environments. The episomal structure of these vectors prevents them from integrating into the host genome which reduces the possibility of insertional mutagenesis.
- Advantages in preclinical studies
The multiple benefits of AdVs make them perfect candidates for delivering genes. They show high transduction efficiency which enables strong gene expression across various cell types including both replicating and non-replicating cells. The capability of AdVs to achieve high transgene expression levels proves essential for medical applications like oncolytic therapy and vaccine production. AdVs can be manufactured and purified in large volumes at high concentrations affordably which makes them ideal for extensive application purposes. The episomal nature of these vectors lowers the potential for insertional mutagenesis which represents a major threat to gene therapy techniques.
- Challenges and limitations
Despite their advantages, AdV face several challenges. Their strong immunogenicity remains a significant hurdle because it triggers severe immune responses especially when high doses of the vector are used. The body's immune response against transduced cells leads to their elimination which in turn reduces the effectiveness of the treatment. The widespread presence of pre-existing adenovirus immunity among humans reduces the effectiveness of adenovirus-based gene therapy methods. Researchers have developed multiple strategies to address these challenges through the implementation of AdVs together with heterologous prime-boost approaches along with immunosuppressive agents. The short-lived transgene expression produced by AdVs restricts their application in situations that need persistent gene expression.
- Case studies: Applications in cell and animal models
Preclinical studies have extensively employed AdVs for multiple applications. In oncolytic therapy scientific advances enable adenovirus vectors to target cancer cells specifically for replication which results in tumor breakdown and elimination. AdVs serve as platforms for antigen expression from multiple pathogens which generate strong immune responses during vaccine development studies with animal models. AdVs represent a significant development in infectious disease vaccine research through their demonstrated strong immunogenic responses and protective effects in preclinical studies. The robust immune response generated by AdVs presents difficulties for moving laboratory successes into clinical treatment applications.
Lentivirus Vectors for Gene Therapy
Structure and characteristics of lentivirus vectors
The human immunodeficiency virus (HIV) stands as the most recognized lentivirus which serves as the basis for constructing LVVs that belong to the retrovirus family. Scientists design these vectors to lose replication ability through the removal of essential viral genes which are then substituted with therapeutic or research-focused transgenes. Lentivirus vectors consist of multiple critical components.
- Transfer Vector:
The genetic payload contained by this component becomes part of the host genome allowing its expression to continue over an extended period. The transgene of interest within this system is positioned between long terminal repeats (LTRs) which enable both reverse transcription and genomic integration. The use of self-inactivating (SIN) LTRs prevents promoter activation in the 3′ LTR post-integration to reduce oncogene activation risk.
- Packaging System:
The system supplies structural elements and enzymes necessary for assembling vectors and integrating genomes. Helper plasmids which encode the gag, pol, and rev genes are provided in trans to stop replication-competent virus formation. The gag gene produces proteins that build the viral capsid structure and the pol gene produces reverse transcriptase and integrase enzymes that transform the RNA genome into DNA before integrating it into the host genome.
- Envelope Protein:
The vector's ability to infect different cell types depends on its envelope protein which defines its tropism. Scientists often use vesicular stomatitis virus glycoprotein (VSV-G) as an envelope protein because it enables vectors to work with various host cells and increases vector stability. LVVs with VSV-G envelopes can be concentrated through ultracentrifugation unlike native HIV envelopes which results in higher potency for both in vivo and ex vivo applications.
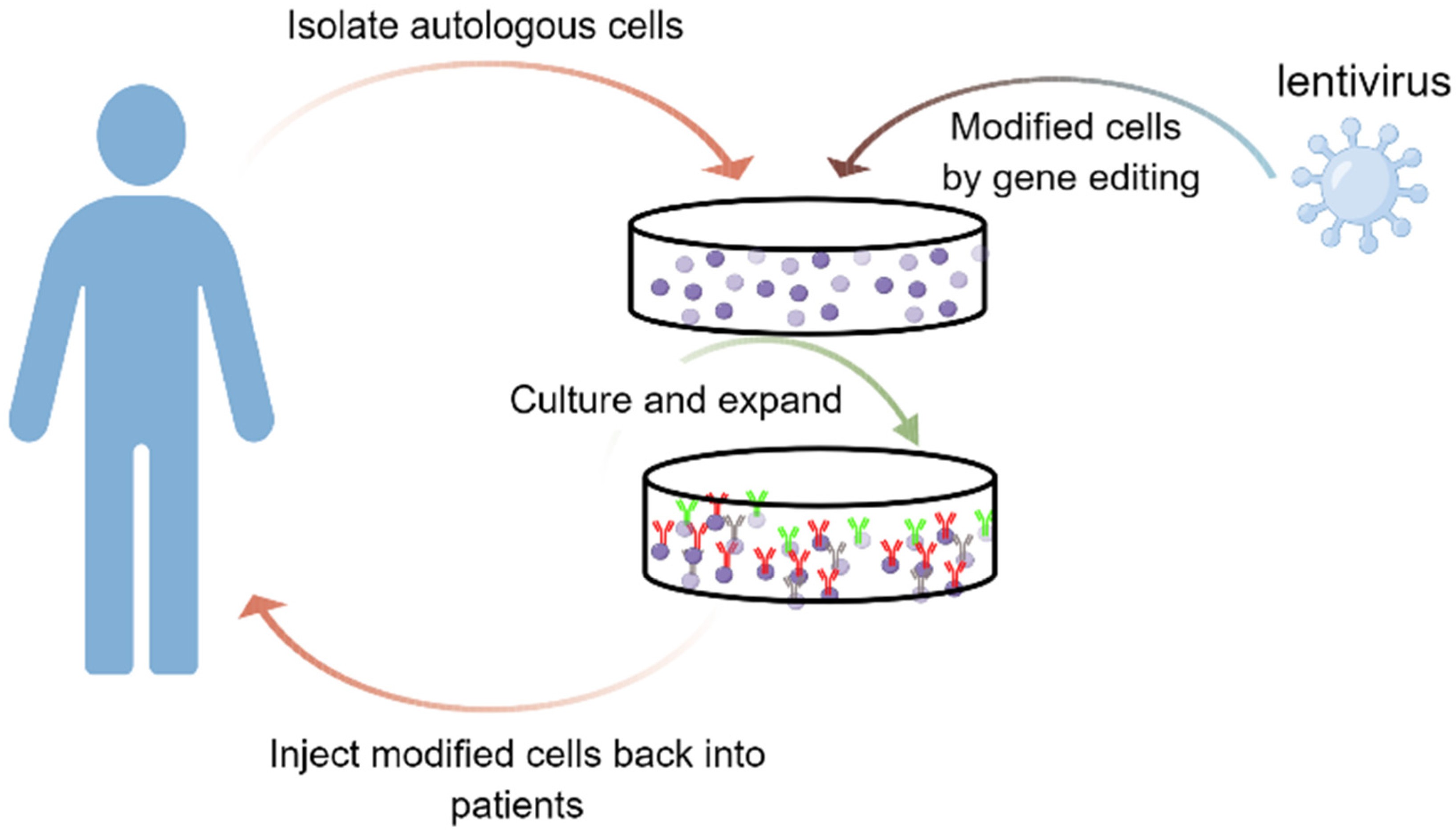 Fig.3 Schematic for the ex vivo strategies using LV vectors for treating genetic diseases2,6.
Fig.3 Schematic for the ex vivo strategies using LV vectors for treating genetic diseases2,6.
Advantages in preclinical studies
- Ability to Integrate into the Host Genome
LVVs provide the significant benefit of integrating therapeutic genes into the host genome. The integration process enables consistent transgene expression over extended periods which is critical for treating chronic diseases that need ongoing genetic correction. In gene therapy for inherited disorders long-term transgene expression remains essential for effective therapeutic outcomes.
- Broad Tropism
LVVs possess the ability to infect multiple cell types including both dividing and non-dividing cells which makes them highly adaptable for diverse experimental applications. The natural infection mechanism of these vectors enables them to penetrate the nuclear membrane of non-dividing cells which results in their broad tropism. The ability to target different tissues and organs enables the broadening of potential applications within gene therapy.
- Large Packaging Capacity
LVVs offer relatively large packaging capacity which enables them to deliver multiple genes or bigger single genes at once. The treatment of complex genetic disorders demands the expression of multiple therapeutic genes or regulatory elements which makes this capability essential.
Challenges and Limitations
- Potential for Insertional Mutagenesis
The primary safety issue related to lentivirus vectors involves their potential to cause genetic mutations through insertional mutagenesis. Viral DNA integration into host genomes might interrupt regular gene activity or turn on oncogenes which could result in harmful conditions like tumor formation. Researchers created SIN LVVs that exclude viral enhancer and promoter elements to lower the chances of accidental genetic disruptions.
- Immune Response
LVVs trigger a weaker immune response compared to adenovirus vectors but repeated or high-dose administrations carry a risk of immune activation. The interaction of the innate immune system with viral components triggers inflammation and immune cell mobilization which affects gene delivery performance and expression levels. Immune responses can be reduced by selecting envelope proteins that provoke lower immune reactions together with temporary immune suppression.
- Production Challenges
The process of scaling up production and refining lentivirus vector purification presents obstacles regarding expansion capacity and cost-effectiveness. The production of high-quality and high-yield lentivirus vectors for clinical use demands both specialized facilities and expert knowledge.
Case studies: Applications in animal models
- Hematopoietic Stem Cell Transplantation
Researchers have used lentivirus vectors widely in preclinical HSC transplantation investigations. The research utilized lentivirus vectors to transfer therapeutic genes into HSCs which subsequently underwent transplantation into animal models. Researchers used lentivirus vectors to introduce functional ADA genes into HSCs in SCID animal models which produced a sustained genetic correction and immune system restoration.
- Neurodegenerative Diseases
Preclinical studies have utilized lentivirus vectors to target neurodegenerative disorders including Parkinson's disease. Researchers used lentivirus vectors in animal models to deliver genes that produce neuroprotective factors and enzymes which synthesize dopamine. The research showed long-term therapeutic gene expression in brain tissue which resulted in better motor function along with decreased neurodegeneration.
- Muscular Dystrophy
Scientists have utilized lentivirus vectors to introduce dystrophin or micro-dystrophin encoding genes into muscle cells within muscular dystrophy models. Research indicates that lentivirus vectors can maintain therapeutic gene expression over time which results in improved muscle function and shows promise for treating muscle degenerative diseases.
Comparative Analysis of AAV, Adenovirus, and Lentivirus Vectors
- Efficiency of gene delivery
AAV vectors demonstrate high transduction efficiency across multiple cell types with particular effectiveness in non-dividing cells like neurons and muscle cells. Transduction efficiency shows variability based on which serotype is applied. The AAV9 serotype delivers high transduction efficiency to the central nervous system (CNS) through intravenous administration. The highest transduction rate achieved with AAV2/1 reached levels between 30%-50% at an MOI of 100 across different lung cancer cell lines.
AdVs demonstrate high transduction efficiency across various cell types. The therapeutic gene expression shows a temporary effect which can be restricted by immune system reactions. Adenovirus vectors no longer represent the preferred choice for some research applications like stroke research because their cytotoxicity and temporary gene expression make them less effective than AAV and lentivirus vectors.
LVVs exhibit superior transduction efficiency in comparison to other vectors particularly when applied to dividing cell types. Stable long-term transgene expression achieved by these vectors is essential for chronic disease treatment. LVVs exhibited transduction efficiency above 50% in lung cancer cell lines at an MOI of 1 which surpassed the performance of any tested AAV serotype. Lentivirus vectors managed to transduce more than 40% of cell lines that showed resistance to AAV infection.
- Safety profiles in preclinical models
AAV vectors typically display high safety profiles. These vectors generate weak immune responses and exhibit minimal insertional mutagenesis risk because they remain episomal. Pre-existing immunity to AAV decreases the effectiveness of the vector.
AdVs possess strong immunogenic properties that trigger intense immune responses and result in transduced cells being eliminated. The immune reaction reduces both the length of time and effectiveness of the gene therapy treatment. The widespread pre-existing immunity against adenovirus diminishes the success rate of adenovirus-based gene therapy treatments.
LVVs have a moderate safety profile. The ability of lentivirus vectors to integrate into the host genome enables sustained gene expression but simultaneously creates the potential for insertional mutagenesis. The creation of SIN LVVs has substantially lowered this risk. Preclinical studies indicate that lentivirus vectors perform well in safety evaluations and produce few negative effects.
- Suitability for different types of gene therapy applications
AAV vectors deliver long-term expression in post-mitotic cells making them ideal for use in CNS or muscle tissue applications. Researchers prefer AAV vectors for in vivo treatments because of their minimal immunogenicity and capability to cross the blood-brain barrier with specific serotypes like AAV9. AAV vectors serve as standard treatment options for inherited retinal diseases and neurodegenerative disorders.
Researchers utilize AdVs when high levels of transient gene expression are necessary for treatments like cancer gene therapy or vaccine production. The capacity of these vectors to target multiple cell types allows them to be used in different applications. Their high immunogenicity combined with temporary expression restricts their application in extended treatment periods.
LVVs provide stable long-term therapeutic gene expression which makes them suitable for hematopoietic stem cell transplantation and chronic genetic disease treatments. These vectors demonstrate high versatility because they can infect both cell types while maintaining a large capacity for genetic material. Researchers utilize lentivirus vectors to treat patients with neurodegenerative diseases and muscular dystrophy.
Future Directions in Vector Development for Gene Therapy
Advances in vector development for gene therapy involve persistent innovation and ongoing improvements. Current research aims to enhance the safety and efficiency of vectors by utilizing advanced manufacturing techniques along with novel vector designs and better purity and quality control measures. The field has progressed through optimized cell culture conditions, advanced bioreactors capabilities, custom vector capsids development, tissue-specific promoter systems and rigorous quality control procedures. Recent scientific investigations focus on developing hybrid vectors that merge different systems' strengths by integrating CRISPR/Cas9 with AAV vectors and creating chimeric vectors to benefit from each platform's unique advantages while overcoming their limitations. Current research prioritizes advancements in vector delivery systems which include immune-evasive engineered capsids and innovative delivery vehicles such as lipid nanoparticles and polymer-based carriers. Researchers are developing new methods to achieve precise tissue targeting while reducing unwanted effects and increasing the effectiveness of treatments. The development of AI, ML, bioprinting, and 3D cell culture models promises to transform vector production and testing. The recent advancements in gene therapy technology show significant potential to enhance treatment efficiency while making therapies more available and cost-effective which will transform the treatment approach for numerous genetic conditions.
References
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes. 2022, 13, 1370. https://doi.org/10.3390/genes13081370.
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. https://doi.org/10.3390/ijms24097736.
- Scarsella, L.; Ehrke-Schulz, E.; Paulussen, M.; Thal, S.C.; Ehrhardt, A.; Aydin, M. Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications. Viruses. 2024, 16, 377. https://doi.org/10.3390/v16030377.
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Telegeev, G.D. Current State of Human Gene Therapy: Approved Products and Vectors. Pharmaceuticals. 2023, 16, 1416. https://doi.org/10.3390/ph16101416.
- Arsenijevic, Y.; Berger, A.; Udry, F.; Kostic, C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics. 2022, 14, 1605. https://doi.org/10.3390/pharmaceutics14081605.
- Distributed under Open Access license CC BY 4.0, without modification.
