The Efficiency Equation-Maximizing Gene Delivery with Viral Vectors in Preclinical Studies
Importance of Efficient Gene Delivery in Gene Therapy
The success of gene therapy relies heavily on efficient gene delivery because this process dictates both the treatment's effectiveness and its safety. Achieving high delivery efficiency allows enough target cells to obtain the therapeutic gene necessary for successful treatment outcomes. Effective delivery mechanisms substantially improve treatment outcomes for diseases such as genetic disorders and cancers which depend on correcting a high proportion of cells. Efficient delivery of genes reduces vector quantity requirements which lowers the risk of immune responses and other side effects linked to high vector doses. The use of viral vectors requires careful consideration due to their potential to initiate immune responses which could hinder therapy effectiveness. Targeted delivery of therapeutic genes to specific cells boosts gene therapy safety by minimizing off-target effects and insertional mutagenesis risks.
Gene therapy viral vectors
The field of gene therapy has seen viral vectors become a central focus in research over the last twenty years. Reverse transcriptase viral vectors enable the production of human neural stem cell (NSC) lines which can be used to repair brain damage in neurological disorders. These vectors faced limitations in delivering genes to non‐dividing cells along with oncogenic risks which made them less common in preclinical stroke studies. Both lentiviral vectors and AAV vectors stand out as ideal choices for achieving stable gene expression. Lentiviral vectors feature double the packaging capacity compared to AAVs but they work better for laboratory treatments in contrast to AAVs which excel at targeting central nervous system treatments in live organisms. Adenoviral vectors enable the transportation of external genes to brain tissue although they produce short-lived gene expression that results in significant cytotoxic effects. Adenoviral vectors now seem outdated for stroke research because AAV and lentiviral vectors offer both sustained expression and safer profiles.
- Adenoviruses (Ad) vector gene therapy
The development of gene transfer methods for mammalian cells led to the common use of adenoviruses (Ad) vectors as delivery vehicles. Non-enveloped viruses with double-stranded DNA genomes can package up to 7.5 kb of foreign DNA molecules. Through engineering processes Ad shuttle vectors now support the insertion of foreign DNA sequences that reach 14 kb in length. First-generation Ad vectors experienced strong immunogenicity even after scientists removed E1/E3 genes from their genome. The immunogenic response was greatly reduced in second- and third generation replication-deficient Ad vectors. Third-generation high-capacity adenovirus vectors known as helper-dependent gutless vectors can hold up to 37 kb of foreign DNA. Scientists have engineered oncolytic adenoviruses that replicate specifically within tumor cells which leads to tumor cell destruction. The development of packaging cell lines enables large-scale production of GMP-grade Ad vectors. Ad vectors enable continuous extrachromosomal transgene expression that persists for a minimum of one year without integrating into the host genome. A follow-up study in non-human primates demonstrated that transgene expression could last up to 7 years while reaching only 10% of peak values and showed no long-term adverse effects.
- Adeno-Associated Virus Vector gene therapy
Adeno-associated virus (AAV) vectors with small non-enveloped single-stranded DNA structures can carry up to 4 kb of foreign DNA but show expanded capacity through fragmented, overlapping or trans-splicing Dual AAV vector designs. AAV vectors typically avoid producing toxic or pathogenic reactions. The repeated use of AAV vectors has led to strong immune responses which decrease their delivery effectiveness and transgene expression ability. Researchers have overcome this challenge by administering different AAV serotypes with each subsequent AAV delivery. Researchers have developed Exo-AAV to facilitate lower AAV doses which lessen the immune response to the AAV capsid protein. Exo-AAV8 vectors have shown effectiveness in achieving long-term gene transfer to the liver. The AAV vector can effectively transduce both dividing and non-dividing cells while typically remaining extrachromosomal but reports show that AAV genes sometimes integrate into the host genome. The incorporation of 28S ribosomal DNA homology sequences into AAV vectors led to a 30-fold increase in AAV integration frequency which could improve genetic disease treatment.
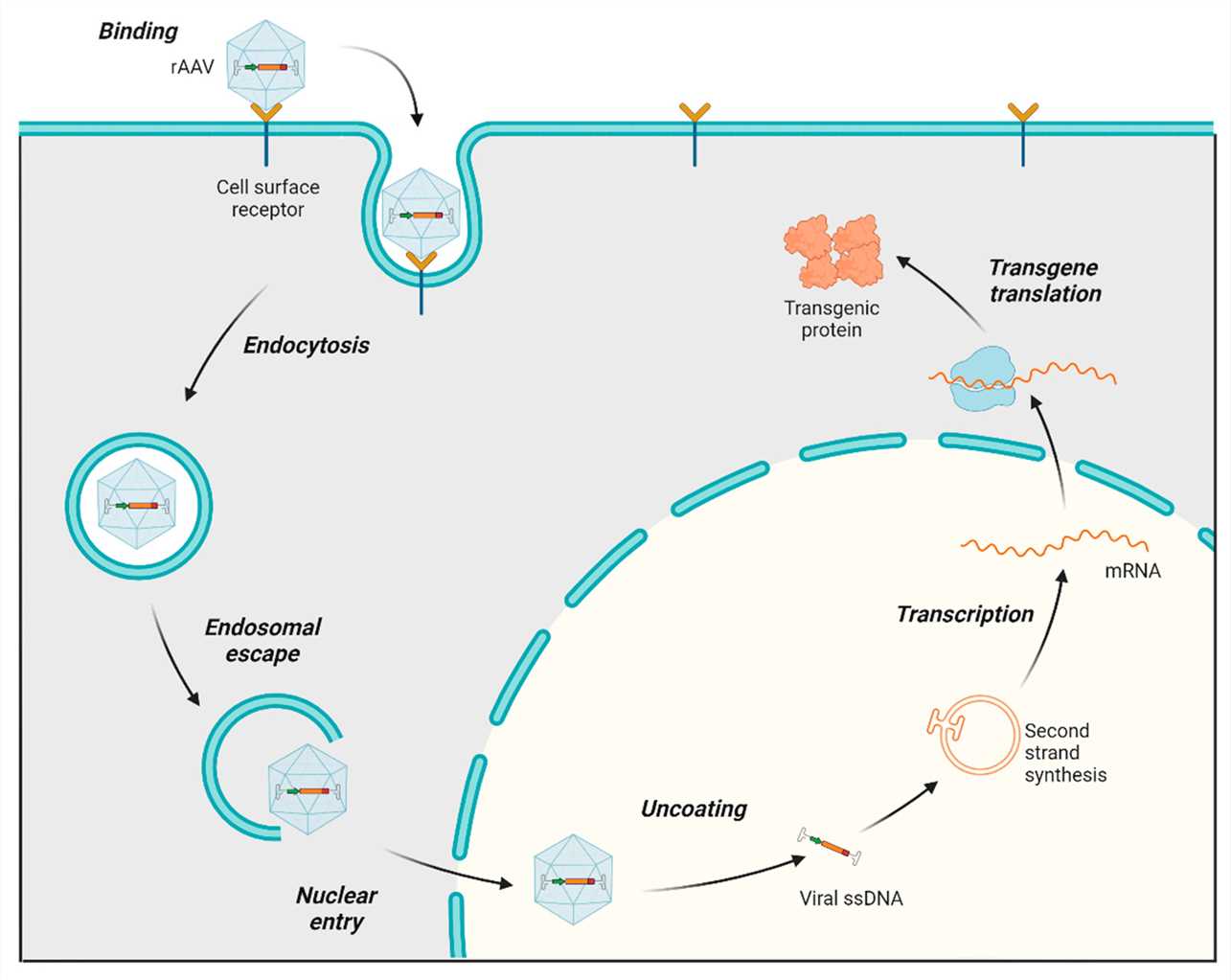 Fig. 1 AAV transduction pathway1,5.
Fig. 1 AAV transduction pathway1,5.
- Herpes Simplex Virus Vector gene therapy
Large herpes simplex viruses (HSV) are enveloped dsDNA viruses that establish latent infections within neural ganglia. Researchers engineered HSV expression vectors and achieved sustained transgene expression. The HSV linear genome creates a circularized viral episome in the nucleus but stays extrachromosomal without integrating into the host DNA. HSV vectors demonstrate exceptional ability to hold over 30 kb of foreign DNA. Engineered HSV amplicons have the capability to package up to 150 kb of foreign genetic material. HSV vectors display strong cytopathogenic effects which scientists have managed to reduce by deleting non-essential genes from the HSV genome. The creation of oncolytic HSV vectors that selectively inhibit cell proliferation in NSCLC cells has occurred through the introduction of micro-RNA sequences (miR-145) into the HSV ICP27 gene. Scientists developed efficient HSV packaging systems including the helper virus-free system which utilizes an ICP27-deleted oversized HSV-1 DNA stored in a bacterial artificial chromosome (BAC) for HSV amplicon production.
- Lentiviral Vector gene therapy
The retroviridae family includes lentiviruses which possess an RNA genome (ssRNA length between 8 to 10 kb) that contains an integrase, reverse transcriptase and protease inside a capsid (p24 protein) and this capsid is enclosed by a host-derived lipid membrane envelope which includes viral glycoproteins which form during the budding stage. Lentiviruses stand out from other retroviruses because they can infect non-dividing cells by transporting their pre-integration complex into the nuclear compartment. The fundamental alteration of lentivirus research involved changing how the virus targets cells. Early studies utilized the native viral envelope that was coded by the viral env gene and demonstrated selectivity for CD4+ cells in HIV-1. Pseudotyping recombinant lentiviral vectors with vesicular stomatitis virus G (VSV-G) glycoprotein enabled their application across various fields due to this glycoprotein's broad cellular tropism.
- Alphavirus Vector gene therapy
Alphaviruses possess an envelope and contain a positive polarity ssRNA genome that can package up to 8 kb of exogenous genetic material. The alphaviruses' positive RNA polarity enables host cell cytoplasm to directly translate viral RNA. Alphaviruses demonstrate a unique RNA self-replication capacity that produces high transgene expression levels. The alphavirus ssRNA undergoes quick degradation which causes a transient nature of expression. Alphavirus vectors function as recombinant particles and operate as either naked RNA replicons, liposome encapsulated RNA replicons or plasmid DNA-based replicons. Scientists created expression systems for Semliki Forest virus (SFV), Sindbis virus (SIN), and Venezuelan equine encephalitis virus (VEE). Both naturally occurring oncolytic M1 viruses and engineered oncolytic SFV vectors have been applied in cancer treatment.
Factors Affecting Vector Efficiency
- Comparison of Transduction Rates and Efficiency
Successful gene therapy outcomes depend heavily on effective gene delivery efficiency. The therapeutic results of gene therapy depend heavily on the differing transduction efficiencies exhibited by various viral vectors. Research comparing different serotypes of AAV and lentivirus vectors demonstrated that lentivirus vectors produced higher transduction rates than AAV vectors. Lentivirus vectors achieved transduction efficiencies exceeding 50% with a multiplicity of infection (MOI) of 1 while AAV2/1 reached its peak transduction rate between 30% and 50% at an MOI of 100. The results indicate that lentivirus vectors display greater efficiency for applications demanding high transduction rates.
- Target Cells and Tissues in Preclinical Models
The selection of target cells and tissues critically affects the success rate of gene delivery systems. The efficiency of gene delivery through preclinical models demonstrates substantial variation based on both the specific cell type and tissue targeted. In lung cancer cell lines AAV serotypes like AAV2/1 demonstrate higher transduction rates than AAV2/4 and AAV2/8. Lentivirus vectors showed better transduction efficiency across several lung cancer cell lines compared to other methods even for cells that resisted AAV infection. Preclinical studies require careful vector selection to match the particular target cells and tissues.
- Delivery Methods and Their Role in Efficiency
Different delivery methods can greatly impact the effectiveness of gene delivery. The efficiency of delivery methods like chemical transfection and electroporation varies along with each method's unique set of challenges. Electroporation achieves effective gene delivery but also leads to substantial cell death which reduces transfection efficiency. Research indicates that chemical transfection techniques like TurboFect result in superior transfection efficiency for particular cell types such as Vero cells when compared to viral vector transduction methods. The specific requirements of gene therapy applications and target cell characteristics must guide the selection of the gene delivery method.
Strategies for Enhancing Vector Efficiency
Genetic Modifications to Improve Transduction Rates
- AAV Capsid Modifications
Scientists have extensively modified Adeno-associated virus (AAV) vectors to increase their ability to deliver genetic material into cells. Changing the capsid proteins through genetic engineering leads to better cellular entry and selective targeting of tissues. Researchers have engineered AAV variants with altered capsid proteins through directed evolution and rational design to avoid existing immunity and improve tissue-specific transduction. The addition of ligands to the capsid surface represents a chemical modification which enhances targeting efficiency while minimizing neutralizing antibody interactions. Chemical modification with N-ethylmaleimide (NEM) on the AAV9 capsid improves bone marrow transduction while decreasing liver transduction.
- Adenovirus Fiber Modifications
The transduction capability of adenovirus vectors has been improved through engineering modifications to their fiber proteins which serve as the receptor-binding elements. These engineered changes boost adenovirus vector cell penetration while lowering the intense immune response typical for these vectors. Scientists have engineered the fiber knob domain so it can target detailed cell surface receptors to boost gene delivery efficiency to specific cells.
- Lentivirus Envelope Modifications
Scientists have engineered lentivirus vectors to enhance their cell targeting abilities and increase their transduction efficiency. Scientists modified envelope proteins including the vesicular stomatitis virus glycoprotein (VSV-G) to extend the vector's ability to infect diverse cell types. Reducing the immunogenicity of the vector through these modifications allows for safer clinical applications.
- Use of Tissue-Specific Promoters for Targeted Expression
The precise targeting of therapeutic genes to specific tissues requires tissue-specific promoters to avoid off-target effects and ensure patient safety.
Neuron-Specific Promoters: Synapsin I promoter functions in neurons and serves as a tool to target the central nervous system. Muscle-Specific Promoters: The promoter for muscle creatine kinase (MCK) functions in skeletal muscle to target gene expression to muscle tissues. Liver-Specific Promoters: The promoter of albumin which functions in hepatocytes serves as the tool for liver-specific targeting.
- Case Studies Demonstrating Promoter Optimization
Research studies have proven that tissue-specific promoters boost vector effectiveness. A study that targeted neurodegenerative diseases utilized the synapsin I promoter in AAV vectors to direct therapeutic gene expression to neurons which led to better treatment results. Targeted expression of therapeutic genes in muscle cells through the use of the MCK promoter in lentivirus vectors led to significant muscle function improvements in muscular dystrophy research.
Optimizing Vector Doses and Administration Routes
- Determining Optimal Vector Doses
To identify the vector dose that provides maximum therapeutic benefit with minimum negative side effects dose-response studies must be conducted. The studies require researchers to give different vector doses to cell cultures and animal models while they evaluate the success of gene transfer and therapeutic results. Research involving mouse models of cystic fibrosis demonstrated that an optimal dose of adenovirus vectors can transduce lung epithelial cells efficiently while triggering minimal immune responses. Multiple case studies have shown how optimizing doses leads to better performance of vectors. Animal model research targeting hemophilia B demonstrated that certain AAV vector doses lead to lasting therapeutic gene expression while avoiding negative side effects. The optimized dose enabled clinical trials to show substantial improvements in clotting factor levels along with fewer bleeding episodes.
- Optimizing Administration Routes
The efficiency of gene delivery depends substantially on the selected route of administration. Typical administration routes for injections consist of intravenous (IV), intramuscular (IM), intracranial (IC), and intravitreal (IVT) techniques. The choice of administration route comes with distinctive pros and cons. Medical professionals use IV administration to target systemic illnesses because it distributes treatment throughout the body whereas IM administration targets muscle tissues because it delivers medication directly into the muscles. The CNS receives treatment through IC administration while IVT administration targets the eye. Multiple research studies indicate that selecting the proper administration route leads to improved vector efficiency. Research on Duchenne muscular dystrophy revealed that AAV vector delivery through intramuscular injection in animal models produced efficient muscle cell transduction and substantial muscle function enhancement. In Parkinson's disease research using animal models researchers found that intracranially delivered lentivirus vectors efficiently transduced neurons which led to better motor function.
- Case Studies: Techniques That Boost Vector Efficiency
Scientists have modified AAV vectors used in neurological disease studies to better penetrate the blood-brain barrier and transduce neurons effectively. Scientists have altered the AAV capsid structure to enhance tissue targeting and minimize immune system reactions. AAV9 vectors underwent chemical modification through NEM to boost their transduction capabilities in the CNS. Optimized AAV vectors demonstrated substantial therapeutic advancements in preclinical research. Studies using a mouse model of SMA revealed that AAV9 vectors delivered the SMN1 gene to motor neurons efficiently which resulted in better motor function and longer survival rates.
Scientists have engineered adenovirus vectors to improve their ability to transduce cancer cells. Scientists have altered fiber proteins to boost their receptor binding potential and cell penetration capabilities. The approach of using tissue-specific promoters enables researchers to direct therapeutic gene expression specifically in cancer cells.
Optimized adenovirus vectors produced significant therapeutic outcome enhancements according to preclinical studies. Adenovirus vectors delivered a tumor suppressor gene to cancer cells in a mouse lung cancer model which resulted in decreased tumor growth and better survival rates.
Balancing Efficiency with Safety in Preclinical Models
- The Challenge of High Efficiency Without Compromising Safety
Preclinical models face the critical challenge of delivering genes efficiently while ensuring patient safety. AAV, adenovirus, and lentivirus serve as potent gene therapy tools but require careful management to prevent negative effects. To achieve therapeutic efficacy transduction efficiency must reach high levels yet it needs to be balanced with safety concerns that prevent immune reactions off-target effects and insertional mutagenesis.
- Preclinical Studies That Demonstrate Successful Balance
Preclinical studies have shown successful achievement of both efficiency and safety balance in gene therapy applications. Self-inactivating vectors demonstrate decreased insertional mutagenesis risks alongside preserved transduction efficiency. The integration of tissue-specific promoters has improved expression precision while reducing unintended effects. Research utilizing AAV vectors for neurological disease models found that capsid protein modifications and neuron-specific promoter usage led to enhanced therapeutic efficiency and improved safety outcomes.
Researchers have optimized adenovirus vectors to increase their effectiveness in cancer therapy. Researchers obtained successful transduction of cancer cells and decreased immune responses through alterations of fiber proteins combined with tissue-specific promoters. Lentivirus vectors have been modified with specialized envelope proteins and tissue-specific promoters to improve transduction efficiency in hematopoietic stem cells without compromising safety.
- Future Directions for Efficient and Safe Vector Development
The main goal of future vector development research will be to improve both the efficiency and safety of the vectors. The development of innovative vector designs which incorporate advanced capsid modifications alongside synthetic biology strategies will serve as a fundamental component. AI and ML technologies will facilitate precise targeting for personalized treatments which will improve both safety and efficiency.
The research community will prioritize creating hybrid vectors by merging the beneficial properties of multiple systems. Hybrid vectors are designed to utilize the high transduction efficiency of viral systems alongside the safety benefits found in non-viral methods. Teams will use bioprinting and three-dimensional cell culture models to form precise preclinical models which improve vector production and delivery optimization.
References
- Zwi-Dantsis, L.; Mohamed, S.; Massaro, G.; Moeendarbary, E. Adeno-Associated Virus Vectors: Principles, Practices, and Prospects in Gene Therapy. Viruses. 2025, 17, 239. https://doi.org/10.3390/v17020239.
- Jamour, P.; Jamali, A.; Langeroudi, A.G. Comparing chemical transfection, electroporation, and lentiviral vector transduction to achieve optimal transfection conditions in the Vero cell line. BMC Mol and Cell Biol. 2024, 25, 15. https://doi.org/10.1186/s12860-024-00511-x.
- Bulcha, J.T.; Wang, Y.; Ma, H. Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther. 2021, 6, 53. https://doi.org/10.1038/s41392-021-00487-6.
- Scarsella, L.; Ehrke-Schulz, E.; Paulussen, M.; Thal, S.C.; Ehrhardt, A.; Aydin, M. Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications. Viruses. 2024, 16, 377. https://doi.org/10.3390/v16030377.
- Distributed under Open Access license CC BY 4.0, without modification.
