Engineering the Future-Customizing Viral Vectors for Enhanced Gene Therapy Efficacy
Viral Vectors for gene therapy
Viruses possess innate capabilities to invade cells according to specific tropism which enables them to breach biological membranes and transport target genes to precise locations making them optimal vectors for gene therapy applications. Different viral vectors have been engineered for gene therapy and cell therapy applications according to specific therapeutic approaches and targeted tissues. Vectors for gene therapy and cell therapy include AAV, lentivirus, retrovirus, adenovirus, herpes simplex virus (HSV), oncolytic viruses, chickenpox virus, measles virus, and Newcastle disease virus.
- AAV vectors
AAV vectors stand out as the most accepted viral delivery tool for rare genetic disease treatment among all options available. AAVs are single-stranded DNA viruses without envelopes that begin their cell entry process by attaching glycan moieties to cellular surfaces across multiple serotypes. Recombinant AAV (rAAV) vectors package essential regulatory elements and the transgene of interest instead of viral genes. For systemic use these vectors show minimal toxicity and immunogenic response yet different serotypes show unique tissue targeting patterns when tested in living organisms. Different serotypes of vectors demonstrate significant potential to deliver genetic material to various tissues and serve as the standard delivery system in ongoing clinical trials. The rAAV serotype rh74 from rhesus monkeys serves as another option because it shows potentially lower immunogenic reactions than human-derived serotypes. AAV's small size restricts it to packaging a linear single-stranded DNA genome of about 4.7 kb thereby limiting the delivery of entire therapeutic genes especially those like dystrophin which have large sizes. The clinical application of AAV faces added obstacles because many humans have developed NAbs against AAV capsids from natural infections.
- Adenoviral Vectors
Researchers first selected adenoviruses for gene therapy studies before any other viruses. Scientists proposed their use as gene delivery vectors two decades ago. These vectors consist of a double-stranded DNA genome that measures 35 kilobases in length. They are nonenveloped viruses. Researchers reduce adenovirus virulence by eliminating genome sections responsible for early protein production. The classification of adenoviral vectors into three generations reflects the different levels of gene deletion used for their attenuation. The first-generation adenoviral vectors exclude both the E1A and E1B genes from their structure. The second-generation adenoviral vectors contain deletions of numerous early genes from their genetic structure. Third-generation adenoviral vectors feature complete removal of the virus genetic material which results in their classification as gutless vectors.
- Lentiviral vectors (LVs)
Lentiviral vectors (LVs) which originate from human immunodeficiency virus demonstrate a substantial packaging capacity of up to 8 kb and maintain low immunogenicity due to their enveloped single-stranded RNA structure. Both dividing and nondividing mammalian cells can be transduced by these vectors because they perform reverse transcription of their RNA genome followed by integration into the host genome. LV-based gene therapies have become a standard treatment modality for hematological disorders through the genetic modification of patient-specific hematopoietic stem and progenitor cells (HSPCs). Recent research indicates that LVs can deliver therapeutic cargo to muscle tissues by using muscle-specific promoters thus expanding their potential use in genetic disease therapies.
Strategies for Vector Customization
Genetic Engineering of Viral Vectors
- Capsid Modification for Enhanced Tropism
Viral vector engineering frequently targets capsid protein modifications to boost infection efficiency in designated cell types. AAV vectors can be altered through genetic modifications of their capsid proteins to enhance their ability to target specific tissues like neurons and muscle cells. The application of directed evolution and rational design techniques enables scientists to create mutations or insertions within capsid genes to strengthen their attachment to particular cell surface receptors. Target cell gene delivery efficiency improves through this method which simultaneously minimizes off-target effects to boost therapy safety and effectiveness.
The chemical modification of the AAV9 capsid using N-ethyl Maleimide (NEM) has been shown to enhance its specificity for murine bone marrow cells and reduce its effectiveness in liver transduction. Researchers utilized site-specific mutagenesis to alter the AAV capsid structure which produced a variant demonstrating increased adipose tissue targeting and eliminated liver tropism.
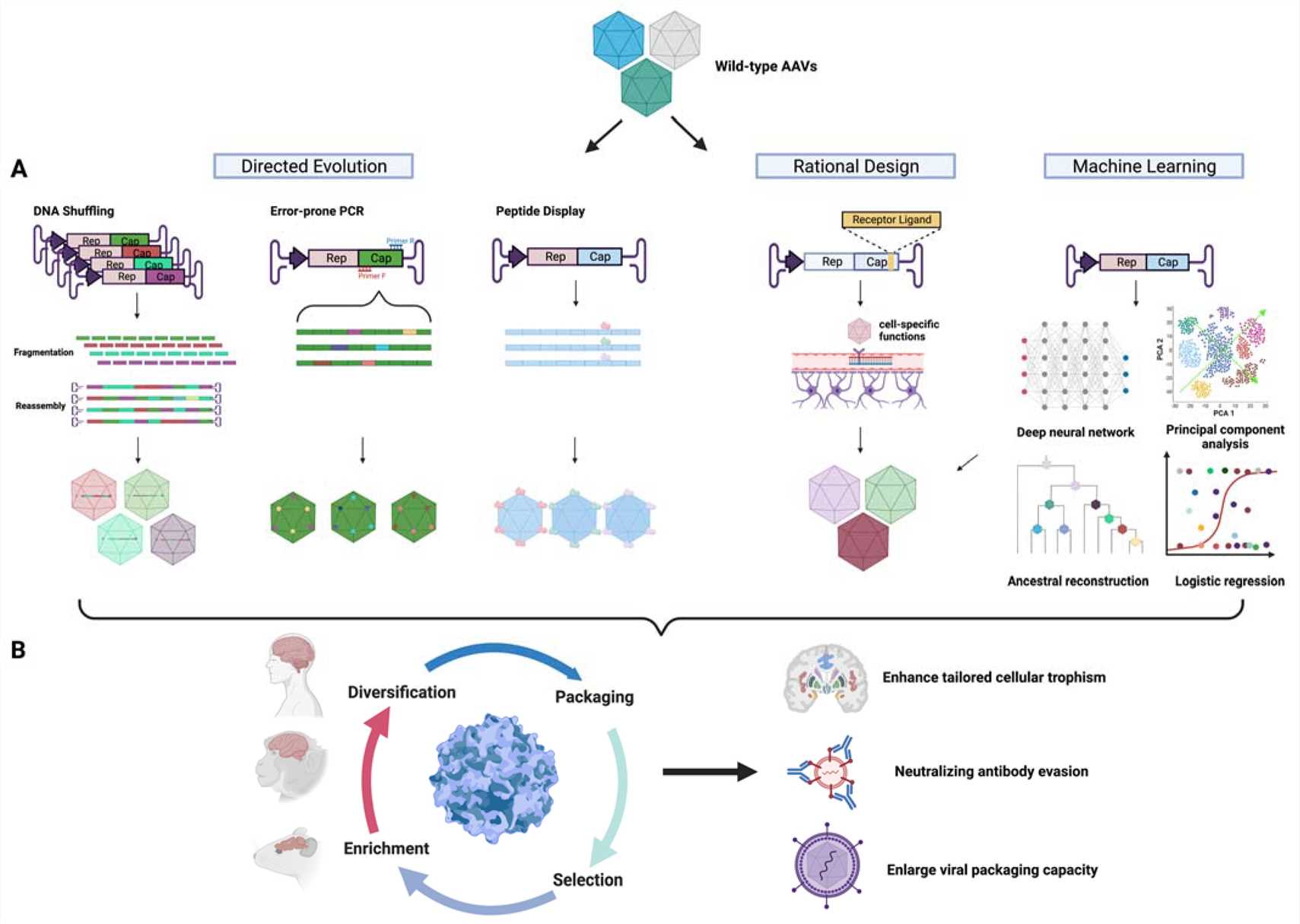 Fig. 1 Schematic diagram of capsid engineering to improve CNS trophism1,5.
Fig. 1 Schematic diagram of capsid engineering to improve CNS trophism1,5.
- Promoter Optimization for Controlled Expression
Selecting the correct promoter determines how effectively the therapeutic gene's expression can be managed. Promoter optimization entails the process of choosing or modifying promoters to direct gene expression exclusively to the target tissue. Tissue-specific promoters enable the therapeutic gene to activate exclusively in targeted cells which in turn prevents unwanted side effects. Inducible promoters enable precise control over gene expression timing by responding to specific stimuli to initiate regulated gene activity.
- Self-Inactivating Vectors for Safety
Researchers created self-inactivating (SIN) vectors to increase the safety of viral vectors. The absence of viral enhancer and promoter elements in the 3' long terminal repeat (LTR) enables these vectors to minimize insertional mutagenesis risk and reduce oncogene activation possibilities.
Chemical and Physical Modifications
- Surface Engineering for Reduced Immunogenicity
Modifying the surface of viral vectors with chemical treatments helps decrease their immunogenic potential. Polyethylene glycol (PEG) coatings on viral vectors enable them to avoid immune detection thereby decreasing the likelihood of immune reactions that can result in vector removal or negative side effects.
- PEGylation for Enhanced Stability
Through PEGylation which involves connecting PEG to vectors' surfaces, researchers can reduce immunogenic reactions while simultaneously improving vectors' durability in the bloodstream. Protecting vectors from degradation with this modification extends their circulation time in the bloodstream which enhances their delivery success to target cells.
- Temperature-Sensitive Mutants for Controlled Release
The implementation of physical modifications through temperature-sensitive mutants enables researchers to achieve extra control mechanisms for gene expression. Scientists design these mutants to activate their therapeutic gene release exclusively at designated temperatures.
Preclinical Testing of Customized Viral Vectors
In Vitro Models
- Cell Line Transduction Efficiency
Researchers depend on in vitro models to assess how effective customized viral vectors perform in transduction processes. Research indicates that the transduction efficiencies of different AAV serotypes vary across multiple cell lines. Research has determined that AAV1 and AAV6 serotypes demonstrate high transduction rates for various human cell lines with efficiencies that surpass 50%. AAV2 demonstrated superior transduction performance specifically in TF1-α cells which are a type of hematopoietic cell line. The results emphasize the necessity to choose the right AAV serotype that matches the specific cell type to achieve maximum transduction efficiency.
- Immune Response Assays
Assays that assess immune response are essential tools for evaluating the potential immune activation risk of engineered viral vectors. These assays evaluate cytokine production and immune cell activation following vector administration. Research shows particular AAV serotypes trigger negligible immune responses which allows their use in situations where immune activation must be minimized. The analysis of different vectors' immune profiles allows researchers to choose the best vector for specific applications while reducing the chances of negative immune responses.
In Vivo Models
- Rodent Models for Safety and Efficacy
Mice and rats serve as common models in preclinical research for testing the safety and effectiveness of tailored viral vectors. These models help investigate how therapeutic genes distribute throughout the body and how effectively they are transferred and expressed over the long term in living organisms. Research with AAV vectors in rodent models demonstrates effective gene transduction and long-term therapeutic gene expression across multiple tissues including liver tissue, muscle tissue, and central nervous system tissue. Scientists utilize rodent models to study potential insertional mutagenesis risks and other safety concerns linked to viral vector integration.
- Large Animal Models for Translational Studies
Translational studies now commonly employ large animal models including non-human primates and pigs as a way to connect preclinical research findings with clinical practice. The models provide a precise depiction of human physiological processes and disease progression while generating important safety and effectiveness data for specialized viral vectors and their pharmacokinetics. Experiments with non-human primates have shown that AAV-mediated gene delivery to the retina is both feasible and safe which enables the transition to clinical trials for patients with inherited retinal conditions. Large animal models help researchers study the long-term stability and expression of therapeutic genes together with potential immune responses and adverse effects.
Case Studies of Customized Vectors
- AAV Vectors in Ocular Gene Therapy
Ocular gene therapy using AAV vectors has demonstrated substantial potential for the treatment of inherited retinal diseases. Researchers recently tested a new AAV variant called AAVv128 which was engineered to improve transduction efficiency and target specificity in retinal tissues.
Subretinal injection of AAVv128 yielded significantly better transduction efficiency in mouse models when compared to AAV8. AAVv128 showed 2-fold higher mean pixel intensity per pixel area at Day 14 and maintained a 1.4-fold increase at Day 28 which demonstrates enhanced transgene expression. AAAVv128 showed a quicker start to transgene expression which demonstrated significant growth from Day 0 until Day 14.
The AAVv128 vector surpassed AAV8 when administered intravitreally and suprachoroidally in both New Zealand rabbits and non-human primates. After intravitreal injection retinal-choroid tissues showed a 12.4-fold increase in vector genome abundance for AAVv128 compared to AAV8.
- Therapeutic Efficacy in nAMD Model
Research in a laser-induced CNV model displayed that AAVv128-anti-VEGF vector suppressed Grade IV lesions in NHPs which indicates its capacity to treat neovascular age-related macular degeneration (nAMD). Grade IV lesions were completely inhibited in the AAVv128-treated group but remained present in 31.25% of cases at day 35 and 41.67% of cases at day 49 for the AAV8-treated group.
Hematopoietic stem cells (HSCs) utilize lentivirus vectors in gene therapy applications because they enable permanent genomic integration which ensures continuous therapeutic gene expression.
Researchers examined how LentiBOOST and prostaglandin E2 (PGE2) together could increase lentivirus vector transduction efficiency in hematopoietic stem cells. The study results demonstrated that LentiBOOST enhanced repopulating HSC transduction efficiency better than PGE2 by itself. Transduction effectiveness remained unchanged when PGE2 was added to LentiBOOST, showing that LentiBOOST alone can adequately enhance HSC transduction.
- Long-Term Stability and Expression
Lentivirus vectors produce sustained therapeutic gene expression in HSCs which makes them an effective tool for treating genetic diseases like severe combined immunodeficiency (SCID) and β-thalassemia. When the lentivirus vector integrates into the host genome it maintains continuous expression of the therapeutic gene which is essential to correct genetic defects over extended periods.
The high transduction efficiency and short-term expression profile of adenovirus vectors make them appropriate for cancer gene therapy applications. The capacity of adenovirus vectors to target both dividing and non-dividing cells makes them perfect for delivering tumor suppressor genes or prodrug-converting enzymes to cancer cells because their high transduction efficiency allows a large portion of cancer cells to acquire the therapeutic gene which boosts treatment effectiveness.
Adenovirus vectors exhibit temporary gene expression which benefits short-term medical applications like cancer therapy because permanent gene expression is unnecessary. The use of transient expression lowers the possibility of insertional mutagenesis and other side effects connected to stable integration. The combination of adenovirus vectors with immune checkpoint inhibitors delivers therapeutic genes through high transduction efficiency while other agents modulate immune responses to boost cancer treatment efficacy.
Challenges and Future Directions
- Overcoming Immune Responses
The immune system's reaction to viral vectors presents a major obstacle for gene therapy applications especially when using AAV vectors in ocular treatments. Though AAV vectors successfully deliver therapeutic genes they generate immune reactions systemically and locally which can cause ocular inflammation. Scientific advancements have introduced multiple approaches that suppress the immune responses and inflammation triggered by AAV vectors. Clinical trials have demonstrated that high doses of vectors result in inflammation which makes reducing vector dose essential. To reduce risk exposure lower doses work while a 1 to 1.5 × 1011 vg/eye dosage achieves effectiveness with safety. The development of AAV vectors that resist neutralizing antibodies (NAbs) demonstrates potential progress in reducing immunogenicity. Researchers have developed synthetic AAV variants which can escape neutralizing sera from multiple species. Studies show that capsids altered through chemical modification display fewer interactions with neutralizing antibodies. The application of DNase treatment to AAV vectors removes extra-viral DNA impurities which lowers innate immune responses while maintaining transduction efficiency.
- Enhancing Vector Stability and Longevity
The success of gene therapy applications in chronic disease treatment depends on achieving stable and long-lasting gene expression. Lentivirus vectors function through genome integration to deliver stable long-term therapeutic gene expression yet their integrity and potency prove difficult to maintain over extended periods. Manufacturing high-quality lentiviral vectors requires a regulated multi-stage process to optimize yield as well as functional performance and safety outcomes. Storage conditions significantly influence vector stability. Vectors are preserved against degradation by storing them at -80°C inside cryoprotectant solutions which include stabilizers such as sucrose or trehalose. Envelop protein adjustments combined with stabilizing viral genome elements through vector engineering lead to enhanced vector longevity and robustness. The improvements made to lentiviral vectors allow them to stay effective for both clinical applications and research purposes throughout extended periods.
- Innovations in Vector Design
Advancements in vector design are crucial to boost gene therapy performance regarding its efficiency and safety while targeting specific genes. The tumor-specificity of adenovirus vectors in cancer treatment improves through changes made to their viral capsid proteins. The use of tumor-specific promoters to control viral replication in cancer cells establishes an effective strategy for treating cancer. Current research for AAV vectors in ocular gene therapy aims to improve both vector specificity and efficiency. Researchers employ directed evolution and rational design to create new capsid variants which enable them to accurately target specific cell populations. The use of AI and machine learning technologies plays a crucial role in developing vectors that demonstrate improved targeting precision and minimized immunogenic responses through rational design methods. The latest progress in vector design shows potential to address present obstacles and increase gene therapy's therapeutic capabilities.
References
- Ghauri, M.S.; Ou, L. AAV Engineering for Improving Tropism to the Central Nervous System. Biology. 2023, 12, 186. https://doi.org/10.3390/biology12020186.
- Araújo, NM.; Rubio, IGS.; Toneto, NPA.; Morale, MG.; Tamura, RE. The use of adenoviral vectors in gene therapy and vaccine approaches. Genet Mol Biol. 2022, 45(3):e20220079. https://doi.org/10.1590/1678-4685-GMB-2022-0079.
- Luo, S.; Jiang, H.; Li, Q. An adeno-associated virus variant enabling efficient ocular-directed gene delivery across species. Nat Commun. 2024, 15, 3780. https://doi.org/10.1038/s41467-024-48221-4.
- Wang, Y.; Shao, W. Innate Immune Response to Viral Vectors in Gene Therapy. Viruses. 2023, 15, 1801. https://doi.org/10.3390/v15091801.
- Distributed under Open Access license CC BY 4.0, without modification.
