The Delivery Dilemma: Overcoming Barriers to Gene Therapy Vector Efficacy in Preclinical Models
Vectors in Gene Therapy
- Non-viral vector
The "vector" particles consist of synthetic biological systems that contain encapsulated therapeutic gene expression cassettes within plasmid DNA (pDNA) attached to synthetic chemicals which get released at their target destinations when delivered. Non-viral systems present a simpler production process than viral-derived vectors and they have a diminished potential to trigger inflammatory responses. Non-viral vectors deliver synthetic compounds such as oligonucleotides or siRNA alongside pDNA which makes them important even though their delivery efficiency is lower compared to viral vectors.
- Lipid-based vectors
The application of lipids as gene delivery systems dates back many years. The positively charged headgroups of most lipids interact electrostatically with nucleic acids' anionic phosphate groups to create lipoplexes. The lipoplexes demonstrate self-assembling lipid tail structures which cause them to form liposomes, solid lipid nanoparticles or lipid emulsions. Lipid carrier materials surpass other options by being biodegradable and less toxic with the ability to contain both hydrophilic and hydrophobic substances. The initial small interfering ribonucleic acid (siRNA) treatment to gain FDA approval called Onpattro used a lipid-based vector.
- Protein-Based Vectors
Gelatin and albumin represent the primary types of protein-based vectors deployed for gene delivery purposes. The strong antigenicity properties of gelatin polymers make them highly suitable for gene delivery applications. Scientists developed nanoparticle vectors made of gelatin to deliver polymerized siRNA. The engineered nanoparticles shielded siRNA genetic material from environmental degradation and effectively targeted melanoma cells in tumor-bearing mice resulting in proper gene expression. Scientists developed a gelatin-based nanocarrier system to transport STAT6 siRNA into A459 cancer cells for STAT6 gene development and expression inhibition. The research demonstrated the ability of gelatin-based nanovectors to achieve effective gene silencing and destruction of A459 cancer cells.
- Polymer-Based Vectors
Scientists created the first polycationic polymer Polyethylenimine (PEI) in 1995 with both branched and linear structures for gene therapy applications. The polymer chain backbone features amine groups in a unique arrangement that limits protonation to only partial levels within physiological pH conditions. The more acidic environment of the endosome leads to protonation of additional amine groups. The charged PEI produces an osmotic response called the "proton sponge effect" which causes endosome bursting and improves transfection efficiency. PEI's high buffer capacity facilitates the release of the gene payload from endosomes. PEI continues to serve as the benchmark standard for evaluating the transfection efficiency of non-viral vectors in current research.
Barriers to Vector Efficacy
- Physical Barriers
The cell membrane poses a major obstacle to delivering genetic material into cells. DNA and RNA nucleic acids are big hydrophilic molecules that find it hard to penetrate the lipid bilayer of cell membranes. Vectors need to either make temporary membrane openings by physical methods like electroporation and sonoporation or activate cellular uptake processes including endocytosis, pinocytosis and phagocytosis to enable entry.
Physical barriers are further compounded by the structural design of tissues. The extracellular matrix (ECM) that surrounds cells acts as an obstruction to the movement of large macromolecules through diffusion. The layers of epithelial and endothelial cells alongside the dense ECM within liver and muscle tissues create substantial barriers that limit vector access to target cells. The blood-brain barrier (BBB) consists of specialized endothelial cells which block substances from moving from the bloodstream into the brain and as a result create difficulties for effective gene delivery to neurons.
- Biological Barriers
The immune system presents a major biological obstacle for successful gene delivery. The innate immune system detects and eliminates viral vectors which causes them to be quickly removed from the body. Adenovirus vectors activate complement systems and produce pro-inflammatory cytokines which leads to reduced vector efficacy through strong immune responses. Pre-existing immunity to viral vectors including AAV can fully prevent transduction regardless of the dose used.
Vectors need to be taken up effectively by target cells after they arrive at their destination. Lipoplexes and polyplexes commonly experience difficulties when they need to penetrate cells effectively. Vector uptake efficiency depends upon its surface characteristics as well as its size and electrical charge. Vectors need to leave endosomes to survive the destruction process in lysosomes. To improve endosomal escape, scientists utilize fusogenic peptides and osmotic agents which break down the endosomal membrane.
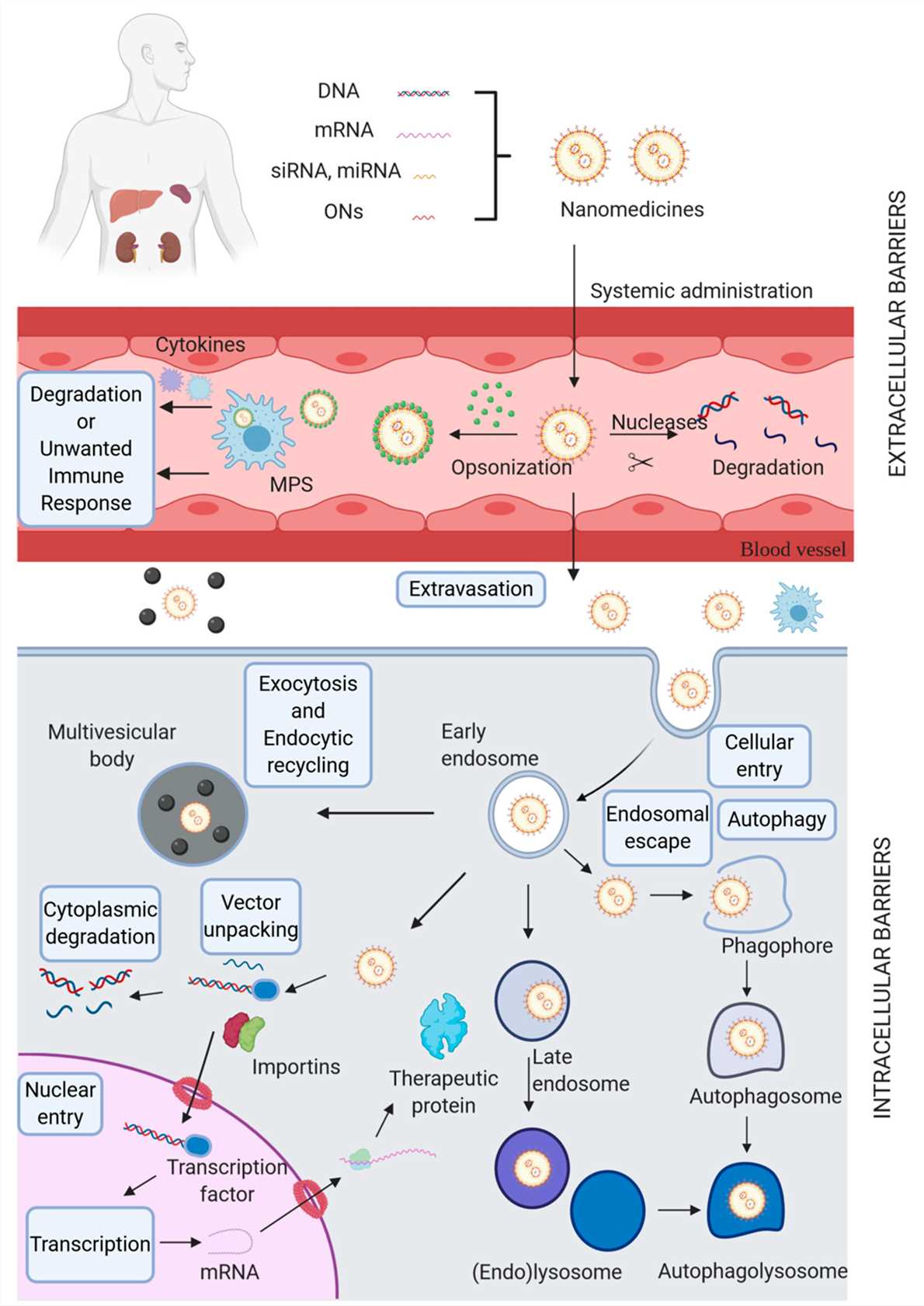 Fig. 1 The biological barriers for non-viral gene delivery systems1,6.
Fig. 1 The biological barriers for non-viral gene delivery systems1,6.
- Impact of These Barriers on Gene Delivery
Gene delivery effectiveness and efficiency depend heavily on physical and biological barriers. Cell membranes along with tissue architecture form physical barriers that restrict vector access to target cells while biological barriers including immune responses and cellular uptake difficulties decrease gene delivery effectiveness. Neutralizing antibodies targeting AAV vectors can prevent transduction entirely in clinical scenarios which underscores the essential requirement to bypass these obstacles.
Strategies to Overcome Delivery Barriers
Vector Modifications to Enhance Delivery
- Surface Modifications
Altering vector surfaces can improve their ability to penetrate physical barriers and escape biological defense systems. Polyethylene glycol (PEG) coatings on viral vectors reduce their immunogenicity while also prolonging their time in circulation. Non-viral vectors modified with cell-penetrating peptides (CPPs) achieve improved cellular entry and enhanced endosome escape capabilities.
- Capsid Engineering
Modifying the capsid proteins in viral vectors leads to better tissue targeting while decreasing their immunogenic response. Researchers have utilized directed evolution and rational design methods to engineer AAV capsids that demonstrate lower immunogenicity and better transduction performance in particular tissues. Lentivirus vectors demonstrate improved infection of non-dividing cells and lowered immune detection when their envelope proteins are modified.
- Genetic Modifications
The inclusion of genetic components that boost vector stability together with expression levels leads to improved delivery efficiency. Self-inactivating (SIN) elements in lentivirus vectors help reduce the risk of insertional mutagenesis. MicroRNA target sequences enable researchers to control gene expression while reducing adverse side effects.
Use of Delivery Vehicles to Improve Efficacy
- Lipid Nanoparticles (LNPs)
LNPs function as lipid carriers which protect nucleic acids from decomposition and facilitate cellular entry. Lipid nanoparticles provide exceptional performance in moving mRNA molecules and siRNA into cells. LNP development focuses on enhancing stability alongside lowering toxicity and achieving precise delivery to tissues. Lipid nanoparticles transported the mRNA responsible for producing the SARS-CoV-2 spike protein which forms the basis of COVID-19 vaccines.
- Polymeric Nanoparticles:
Biodegradable polymers form polymeric nanoparticles that encapsulate genetic material for protection. The design of nanoparticles enables controlled payload release which results in prolonged gene expression activity. Targeting ligands can be added to these nanoparticles to improve their specificity towards specific cell types.
- Hybrid Delivery Systems:
By merging multiple delivery systems scientists can utilize each system's specific advantages. The combination of viral and non-viral elements in hybrid vectors enhances transduction efficiency and decreases immunogenicity. Researchers use viral vectors to deliver genes initially and then employ non-viral vectors to maintain gene expression over time.
- Physical Methods:
The use of physical techniques like electroporation, sonoporation, and magnetofection to deliver genetic material works by temporarily forming pores within the cell membranes. Physical delivery techniques show high success rates when targeting muscle and brain tissues which normally resist transfection.
Case Studies: Successful Strategies of Gene Therapy Vector in Preclinical Models
- Animal Models to Test Delivery Optimization
The optimization of gene therapy vector delivery relies heavily on the use of animal models. The evaluation of gene therapy effectiveness for cystic fibrosis (CF) research relies heavily on ferret and pig models. The models deliver important information regarding both the success rate of gene delivery and the sustained expression levels of therapeutic genes within airway epithelial tissues. Researchers investigated recombinant adeno-associated virus (rAAV) vectors in ferret and pig models during a CF study. The research validated the capacity of rAAV vectors to effectively transduce airway epithelial cells which corrected CF-ion transport abnormalities although airway secretions and post-entry barriers emerged as limiting factors for transduction efficiency in older animals. Researchers improved transduction efficiency by evolving rAAV vectors which showed enhanced tropism for pig airways to address these challenges. CF models also incorporate lentiviral vectors for research applications. Scientists used influenza virus hemagglutinin to pseudotype lentiviral vectors which integrate into the host genome to achieve better targeting of airway epithelial cells. Animal-based preclinical studies showed effective transduction of airway tissues in ferrets and pigs while pig models displayed substantial correction of CF-related abnormalities.
- Cell Models to Study Vector-Cell Interactions
Scientists depend on cell models to examine how gene therapy vectors interact with target cells. 3D organoids and microfluidic systems as advanced cell culture methods create physiologically accurate conditions for studying vector-cell interactions. Human airway epithelial cells grown at an air-liquid interface served as a model to test the effectiveness of rAAV vectors for CF gene therapy. Research demonstrated that different rAAV serotypes including AAV1, AAV2, and AAV5 achieved various levels of effectiveness when targeting airway epithelial cells. Optimization of vector design and delivery conditions relied on insights obtained from these cell models.
Future Directions for Overcoming Delivery Challenges
- Emerging Technologies for Enhanced Delivery
The field of gene therapy delivery methods continues to evolve quickly with a focus on better delivery efficiency and safety improvements. LNPs stand out as a major breakthrough in gene delivery technology because their capacity to carry nucleic acids including mRNA and CRISPR/Cas9 components has been proven effective. LNPs protect genetic material from degradation during delivery and enhance cellular uptake which makes them extremely useful for mRNA vaccine delivery. Biodegradable polymer use by scientists enables the construction of polymeric nanoparticles that protect genetic material and enhance cellular penetration while reducing immune system reactions. Scientists are developing a new biotherapeutic delivery technique across the BBB through receptor-mediated transcytosis (RMT). Through combining antibody engineering with BBB-selective RMT target identification scientists developed new brain-penetrating biotherapeutics that show enhanced performance and safety profiles. These advancements play an essential role in treating CNS disorders because the BBB creates a major obstacle to effective drug delivery.
- Potential for Combination Therapies to Improve Efficacy
Using multiple therapeutic agents or methods together in combination therapies greatly boosts the chances for successful gene therapy outcomes. The immune response to cancer cells improves when gene therapy combines with immunotherapy. Preliminary research reveals that therapeutic gene delivery combined with immune checkpoint inhibitors leads to improved tumor reduction and increased survival outcomes. Research shows that pairing gene therapy with small molecule drugs presents a promising treatment approach. Gene therapy enables the delivery of functional genes for genetic disorder treatment while small molecules help regulate the therapeutic gene's expression and target disease-related pathways.
- Innovations in Vector Targeting and Delivery Mechanisms
Developing precise delivery methods through vector targeting innovations represents a key solution to current delivery obstacles. A strategy for improving gene delivery precision involves tissue-specific promoters and targeting ligands. The inclusion of ligands that attach to specific cell surface receptors enables vectors to reach target cells more effectively which minimizes off-target impacts and boosts therapeutic success. The advancement of SIN vectors along with non-integrative vectors represents an innovation aimed at minimizing insertional mutagenesis risk. The absence of viral enhancer and promoter elements in SIN vectors lowers the risk of oncogene activation. Research is underway to improve gene therapy vector stability and delivery through the application of biodegradable polymers and lipid nanoparticles. These materials ensure genetic material remains intact and enable cellular uptake which improves the efficiency of gene delivery.
Comparative Analysis: Delivery Efficacy of Different Vectors
- Transduction Rates and Efficiency
The specific requirements of the target tissue and the therapeutic application must guide the comparison of transduction rates and efficiency among different vectors. Research shows that viral vectors including AAV and lentivirus achieve superior transduction efficiency than non-viral vectors which consist of lipoplexes and polyplexes. AAV vectors demonstrate high transduction efficacy across diverse cell types including neurons and muscle cells and achieve transduction rates above 50% in preclinical studies. Lentivirus vectors exhibit high transduction efficiency in dividing cells which makes them well-suited for hematopoietic stem cell transplantation.
Although non-viral vectors demonstrate lower transduction efficiency, they present benefits through diminished immunogenicity and enhanced scalability. LNPs and polymeric nanoparticles demonstrate potential in genetic material delivery through transduction rates that fall below those of viral vectors yet meet the needs of specific applications. Researchers have successfully used LNPs to transport mRNA for protein replacement therapy applications as shown by efficient cellular uptake and gene expression in preclinical studies.
- Long-Term Expression and Stability
Successful gene therapy depends heavily on both the stability and longevity of gene expression. Lentivirus and AAV stand out among viral vectors due to their capacity for stable long-term therapeutic gene expression. Lentivirus vectors achieve their integration into host genomes which allows them to maintain continuous gene expression for prolonged periods necessary for chronic genetic disease therapies. The AAV vectors function as episomes by default but achieve long-term gene expression in non-dividing cell types like neurons.
Non-viral vectors produce temporary gene expression suitable for treatments that require short-term effectiveness or situations where multiple doses can be administered. LNPs and polymeric nanoparticles effectively deliver mRNA or siRNA to achieve temporary therapeutic effects which work well for vaccine development and short-term gene silencing.
- Suitability across Various Gene Therapy Modalities
Different gene therapy applications require specific vector characteristics which determine the optimal vector choice. Applications that require both high transduction efficiency and sustained gene expression typically favor viral vectors as the delivery system of choice. The effectiveness of AAV vectors in delivering genes to neurons and muscle cells while maintaining stable gene expression makes them a common choice for preclinical and clinical investigations into neurological disorders, muscular dystrophy, and inherited retinal diseases.
Lentivirus vectors represent the optimal choice for hematopoietic stem cell transplantation because stable long-term therapeutic gene expression is essential to correct genetic defects in dividing cells. When treating neurodegenerative diseases researchers utilize vectors that integrate into the host genome to maintain continuous expression of protective genes.
Applications requiring reduced immunogenicity and greater scalability benefit from non-viral vectors like LNPs and polymeric nanoparticles. LNPs demonstrate potential as delivery vehicles for mRNA-based protein replacement treatments as well as CRISPR/Cas9 gene editing tools. Polymeric nanoparticles serve as delivery vehicles for plasmid DNA and siRNA and provide adaptable systems which can be designed for particular uses.
References
- Torres-Vanegas, J.D.; Cruz, J.C.; Reyes, L.H. Delivery Systems for Nucleic Acids and Proteins: Barriers, Cell Capture Pathways and Nanocarriers. Pharmaceutics. 2021, 13, 428. https://doi.org/10.3390/pharmaceutics13030428.
- Bhat, M.; Tharmatt, A.; Bhargava, S. Can breakthroughs in dermal and transdermal macromolecule delivery surmount existing barriers and revolutionize future therapeutics?. J Transl Med. 2025, 23, 513. https://doi.org/10.1186/s12967-025-06219-6.
- Qie, B.; Tuo, J.; Chen, F.; Ding, H.; Lyu, L. Gene therapy for genetic diseases: challenges and future directions. MedComm. 2025, 6:e70091. https://doi.org/10.1002/mco2.70091.
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics. 2020, 12, 225. https://doi.org/10.3390/pharmaceutics12030225.
- Distributed under Open Access license CC BY 4.0, without modification.
