The Future of Gene Therapy Vectors: Innovations and Preclinical Applications
Viral vectors for gene therapy
- AAV vector gene therapy
Adenovirus-related virus (AAV) is a nonpathogenic parvovirus comprising a single-stranded DNA molecule of 4.7-kb inside a non-enveloped icosahedral capsid that ranges from 20 to 25 nm across. The viral genome includes three genes called Rep, Cap and AAP which are flanked by inverted terminal repeats (ITRs) functioning both as replication origins and packaging signals. The Rep gene produces four nonstructural proteins needed for viral genome replication and transcriptional regulation as well as packaging. The three structural proteins VP1, VP2 and VP3 encoded by the Cap gene combine to build a viral capsid that is composed of 60 units. The AAP protein is produced by the aap gene which exists within an alternate reading frame that overlaps with the cap gene. The assembly-activating protein (AAP) enables the nuclear import of capsid proteins and assists in capsid assembly and maturation. A new study reviewing 12 variations of AAV serotypes demonstrated that the assembly activating protein (AAP) does not play a crucial role in the assembly of AAV serotypes 4, 5, and 11. Researchers use AAV vectors extensively in vivo for gene replacement studies to evaluate new therapies in animal disease models and to analyze gene function or silence gene expression.
- Adenovirus vector gene therapy
Adenoviruses (Ads) possess a non-enveloped structure and exhibit an icosahedral protein capsid with a diameter of 90 nm which contains a double-stranded DNA genome. The genomic DNA of Ad spans 26 to 45 kb and includes two ITR flanking sequences. The adenovirus infection process starts when the adenovirus fiber protein attaches to the coxsackie and adenovirus receptor (CAR) while the most frequently used serotype (Ad5) utilizes cell-surface integrin αVβ5. After internalization through endocytosis the viral capsid undergoes partial disassembly before being transported to the nucleus with the help of the dynein/dynactin motor complex. Microtubule motor kinesin-1 breaks down the NPC-docked capsids and the NPC structure to allow the viral genome to enter the nucleus. The viral genome of Ad stays as an episome in the nucleus of host cells. Ad vectors serve as popular vaccine carriers because they efficiently deliver foreign DNA into target cells while naturally triggering host immune responses. Oncolytic replication-competent Ad represents a promising viral vector for cancer treatment.
- Lentiviral vector gene therapy
Scientists commonly create lentiviral vectors using human immunodeficiency virus (HIV), feline immunodeficiency virus (FIV), and equine infectious anaemia virus (EIAV) as their sources. Initially, lentiviral vectors were packaged using three plasmids: The original packaging system consisted of three plasmids which included a vector plasmid that held the transgene between HIV LTRs together with a packaging plasmid containing gag and pol and accessory genes and an envelope plasmid to express the env gene. The most recent lentiviral packaging systems now use four distinct plasmids to increase safety these systems separate plasmids for rev, gag and pol from those carrying the transgene and the envelope encoding function. The elimination of tat is achieved by adding a chimeric 5’LTR and a heterologous promoter to the vector plasmid. The envelope glycoprotein dictates the retroviral and lentiviral tropism which leads scientists to pseudotype recombinant vectors with envelope glycoproteins from different enveloped viruses. The vesicular stomatitis virus glycoprotein G (VSV-G) serves as a common envelope for pseudotyping retrovirus and lentivirus vectors while binding to a universal membrane phospholipid that facilitates cell entry thereby resulting in wide tropism for the vector. Various alternative envelope proteins serve as tools to modify how cells are targeted in living organisms.
Non-viral vectors for gene therapy
- Lipid-based vectors
Among non-viral gene carriers, lipid-based vectors remain one of the most commonly utilized types. In 1980, researchers demonstrated that liposomes made from phosphatidylserine could capture and transport SV40 DNA into monkey kidney cells. A subsequent study demonstrated superior transfection results using synthetic cationic lipid DOTMA which formed small uniform liposomes able to efficiently encapsulate and deliver DNA to multiple mammalian cell lines. Cationic lipids such as DOTMA are characterized structurally by three components: The three structural components of cationic lipids such as DOTMA include a cationic head group and a hydrophobic tail which are connected by a linking group. Several cationic lipids for liposomal gene delivery like DOSPA, DOTAP, DMRIE and DC-cholesterol possess unique structural modifications of their three components. The liposomal formulations contain neutral lipids such as fusogenic phospholipid DOPE and cholesterol which act as 'helper lipids' to improve transfection activity and nanoparticle stability.
- Polymeric DNA vectors
Cationic polymers represent a different kind of non-viral DNA vectors that draw attention because of their extensive chemical variability along with their ability to undergo functionalization. The first polymeric DNA vectors that emerged were poly(L-lysine) (PLL) and polyethylenimine (PEI). The homopolypeptide PLL consists entirely of the basic amino acid lysine and researchers have recognized its DNA condensation capabilities since the 1960s. Initial research from the late 1980s showed that PLL linked with asialoorosomucoid glycoprotein could serve as a non-viral vehicle for gene delivery targeting the liver. Without a lysosomal disrupting agent like chloroquine PLL demonstrates limited transfection activity because its amine groups remain positively charged at physiological pH which limits its ability to buffer and disrupt endosomes. The unmodified form of PLL demonstrates significant cytotoxic effects during in vitro testing. Researchers have reported multiple modified PLL variants that exhibit improved gene delivery capabilities. PLL coated with the hydrophilic polymer PEG works to reduce unwanted interactions with serum components thereby extending its circulation time.
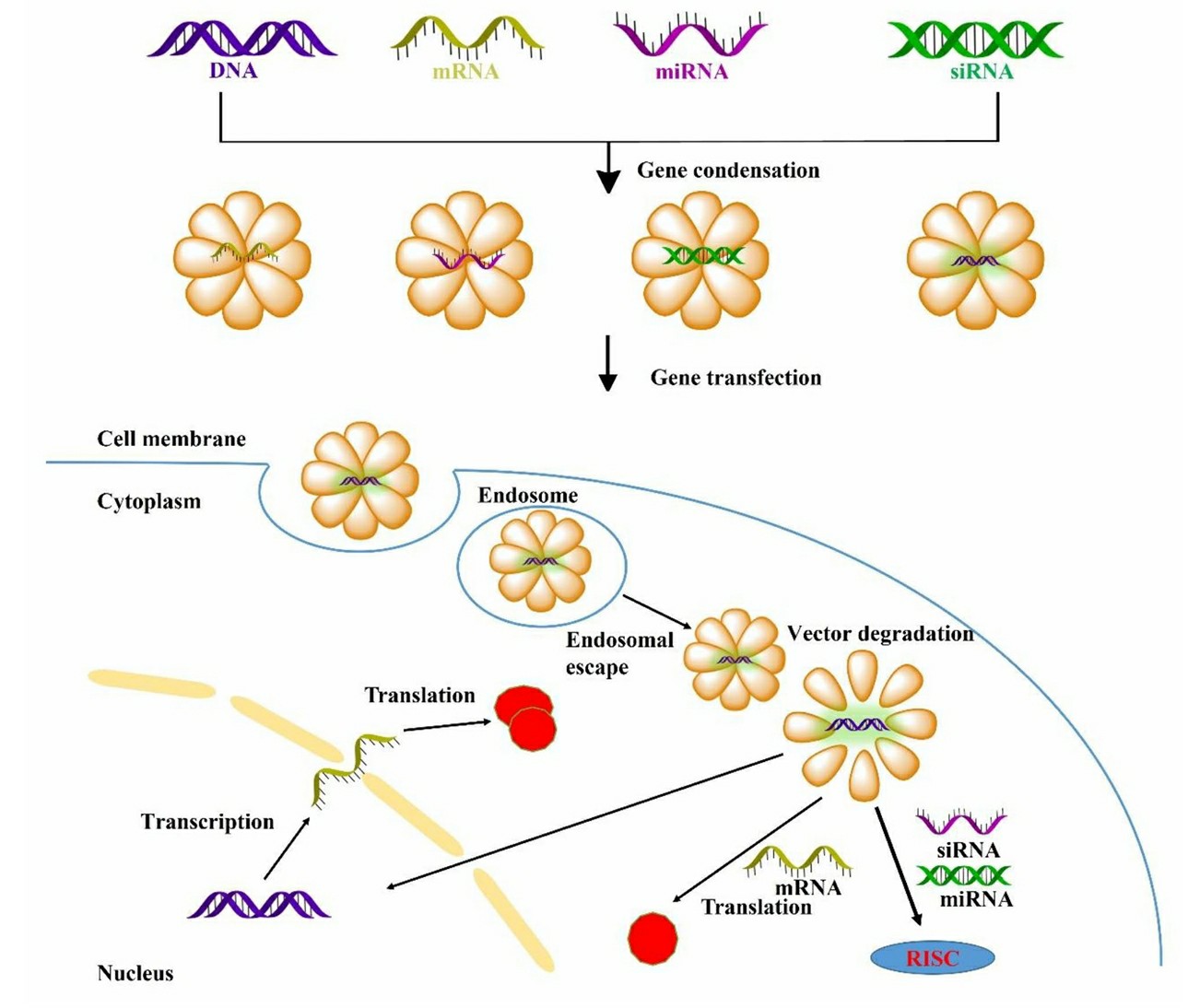 Fig.1 Non-viral vector mediated various gene transfection process, and navigating extracellular and intracellular obstacles for DNA, mRNA, siRNA, miRNA1,6.
Fig.1 Non-viral vector mediated various gene transfection process, and navigating extracellular and intracellular obstacles for DNA, mRNA, siRNA, miRNA1,6.
Emerging Vector Innovations
- New Viral Vector Platforms
Hybrid vectors integrate the advantages of multiple viral systems to address the specific limitations found in single vectors. Hybrid AAV-lentivirus vectors utilize AAV's high transduction efficiency and low immunogenicity alongside lentivirus vectors' stable integration and extended expression capabilities.
Scientists are developing new AAV vectors that tackle issues related to pre-existing immunity and restricted packaging capability. The new generation of AAV vectors encompasses engineered capsids that show lower immune responses and better target specific tissues along with dual AAV systems designed for large gene delivery and self-complementary AAV (scAAV) which enables swifter and more efficient gene expression. Preclinical research indicated that AAV8 vectors with double-stranded self-complementary (sc) genomes achieve therapeutic factor IX (FIX) concentrations in hemophilia B models. The decrease in expression levels over time demonstrates a requirement for additional optimization steps.
Modified lentivirus vectors have been developed to improve safety standards while minimizing insertional mutagenesis risks. Research advancements have introduced self-inactivating (SIN) vectors along with CRISPR/Cas9 technology for precise integration and non-integrative lentiviral vectors (NILVs) as solutions to minimize insertional mutagenesis risks. Preclinical studies show these vector modifications can enhance safety profiles of lentivirus vectors but still provide effective gene delivery.
- Non-Viral Vector Advancements
Researchers are developing advanced nanoparticles to boost both efficiency and precision in non-viral gene delivery systems. Biodegradable polymer-based nanoparticles protect genetic material while improving cellular absorption. Gold nanoparticles deliver genetic material by using physical techniques like electroporation.
Lipid Nanoparticles (LNPs) function as lipid-based delivery systems which hold promise in transporting genetic material including mRNA and plasmid DNA through advances that enhance stability and minimize toxicity while adding ligands or antibodies for targeted delivery. Research studies with animals have shown that LNPs enhance both delivery precision and effectiveness when used for genetic transfer in central nervous system applications.
The revolutionary gene editing capabilities of CRISPR/Cas9 have prompted current research efforts to develop more efficient delivery methods for CRISPR components. The field has developed viral delivery methods for CRISPR components through lentivirus and AAV vectors besides non-viral delivery systems such as LNPs and additional alternatives. Preclinical studies reveal that these delivery methods can produce targeted and effective genetic modifications across multiple cell types and tissues.
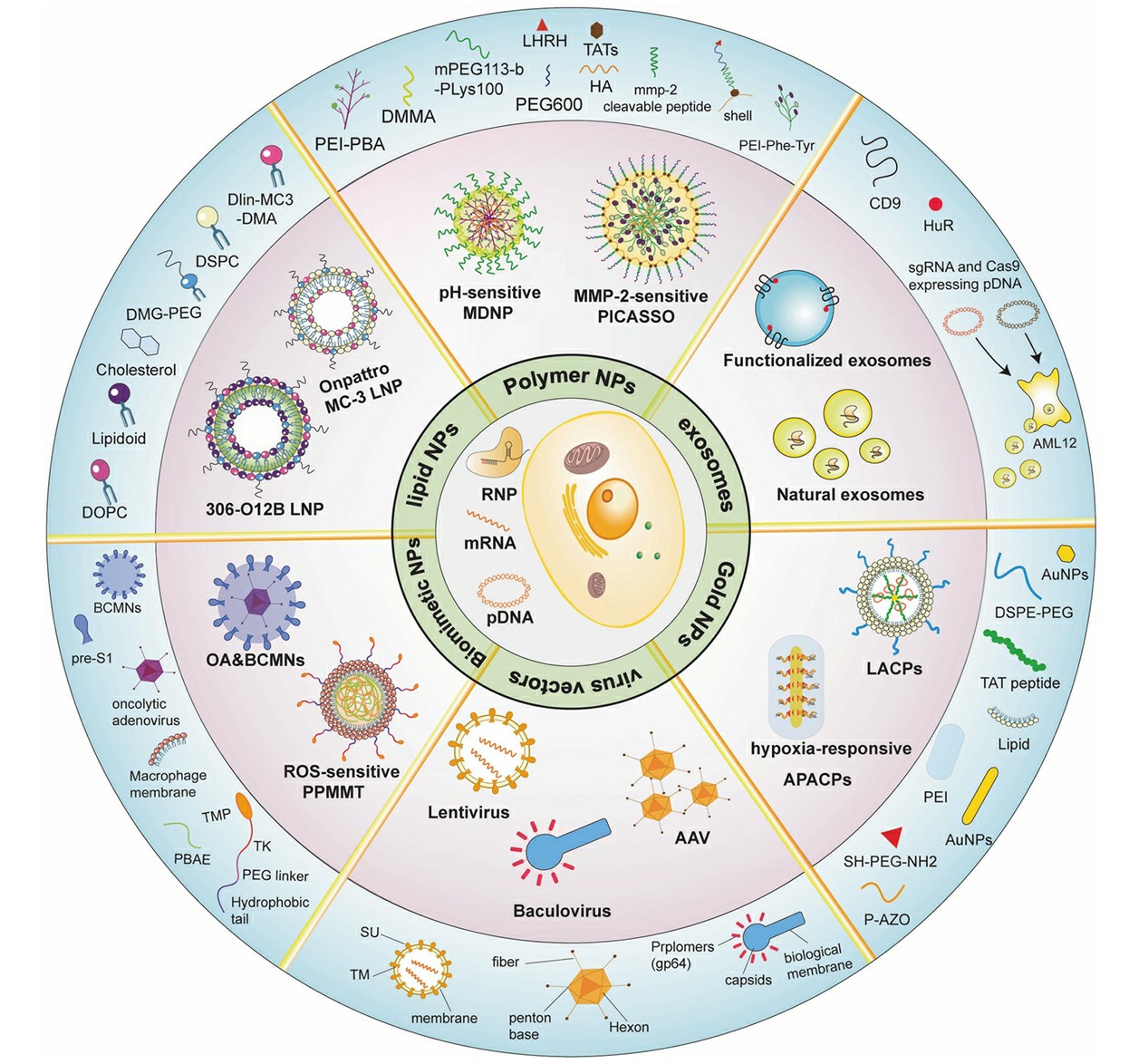 Fig.2 Schematic diagram showing multiple types of vectors for the in vivo delivery of CRISPR systems2,6.
Fig.2 Schematic diagram showing multiple types of vectors for the in vivo delivery of CRISPR systems2,6.
Gene Editing Tools as Vectors
- CRISPR/Cas9 and Other Gene Editing Systems
The CRISPR/Cas9 system enables precise genomic modifications by creating targeted double-strand breaks (DSBs) at specific locations. Researchers employ this system extensively in basic research studies and therapeutic treatments. The process of generating DSBs within the genome can result in unintended mutations together with off-target effects. Researchers have created multiple CRISPR/Cas9 variants along with delivery systems to solve these existing challenges. LNPs serve as delivery vehicles for CRISPR/Cas9 components in vivo which improves both the efficiency and specificity of genome editing techniques.
- Base Editors and Prime Editors
Base editors constitute advanced gene-editing instruments which enable exact single-base substitutions while avoiding DSB formation. There are two main types of base editors: Cytosine base editors (CBEs) and adenine base editors (ABEs) represent the two primary types of base editors available for genome editing applications. These base editing tools enable the transformation of cytosine (C) into thymine (T) and adenine (A) into guanine (G). The methodology lowers the chance of unexpected genetic alterations while proving essential for repairing point mutations that cause hundreds of genetic disorders.
Prime editors (PEs) represent a novel gene-editing platform that merges the capabilities of CRISPR/Cas9 with reverse transcriptase. The ability of prime editors to insert, delete, and replace genetic sequences greatly increases gene editing possibilities. Researchers developed PE2 and PE3 to improve both the editing efficiency and specificity of gene editing techniques.
- Clinical Applications and Future Directions
Preclinical models demonstrate the promise of gene-editing tools for treating different genetic disorders. Base editors successfully corrected precise point mutations in disease models while maintaining minimal off-target effects. Both CRISPR/Cas9 systems and prime editors exhibit the capacity to facilitate intricate genetic modifications.
Despite progress, researchers must still address multiple obstacles such as delivery efficiency enhancement, off-target effect reduction, and maintenance of stable gene editing results over time. Current scientific efforts aim to refine these genetic tools while creating new delivery systems to improve their clinical usefulness.
Preclinical Applications of Innovative Vectors
- Hybrid Vectors in Cancer Gene Therapy Models
Researchers are studying hybrid vectors that merge various viral systems to improve cancer gene therapy effectiveness and safety. AAV-lentivirus hybrid vectors utilize AAV's high transduction efficiency and low immunogenicity alongside lentivirus vectors' stable integration and long-term gene expression capabilities. The application of hybrid vectors in preclinical cancer models demonstrated their potential to target tumor cells with therapeutic genes while simultaneously minimizing immune responses and off-target effects.
Research shows that hybrid vectors can deliver tumor suppressor genes or prodrug-converting enzymes directly to cancer cells resulting in significant tumor shrinkage in animal studies. Hybrid vectors that incorporate tissue-specific promoters and self-inactivating (SIN) elements demonstrate reduced insertional mutagenesis and off-target effects which improves their safety profile for cancer gene therapy applications.
- Next-Generation AAV Vectors in Neurological Disease Models
The development of next-generation AAV vectors seeks to overcome issues related to pre-existing immunity and restricted packaging capacity. The next-generation AAV vector set contains engineered capsids with decreased immune responses and improved tissue targeting abilities together with dual AAV vectors which facilitate the delivery of bigger genetic material and scAAV that achieves quicker and more effective expression of transgenes. Experimental neurological disease models demonstrate that advanced AAV vectors deliver therapeutic genes to the central nervous system effectively. Next-generation AAV vectors demonstrate efficacy in delivering therapeutic genes to CNS neurons and glial cells through preclinical research that confirms their ability to maintain long-term gene expression. Engineered AAV capsids with reduced immunogenicity trigger less immune activation than traditional AAV vectors which enables multiple treatments and long-lasting therapies for neurological disease research in animals.
- CRISPR Delivery Systems in Genetic Disorder Models
The emergence of CRISPR/Cas9 along with other gene-editing technologies has revolutionized gene therapy by enabling precise and efficient genetic mutation correction. Preclinical genetic disorder models have seen improved precision and efficiency in gene editing through CRISPR delivery systems that employ both viral vectors and non-viral nanoparticles. The use of AAV viral vectors for delivering CRISPR/Cas9 systems has shown successful precise correction of genetic mutations in animal models for cystic fibrosis and muscular dystrophy. The latest preclinical research shows that LNPs and base editors improve the safety of CRISPR gene editing through reduced off-target effects.
Challenges of Vectors in Gene Therapy
Challenges in Vector Production and Purification
- Scalability and Yield
The production of viral vectors at large volumes continues to represent a substantial difficulty. AAV vectors produce lower titers than other vectors making scalability more difficult to achieve. The downstream processing of lentiviral vectors encounters difficulties in both large volume management and vector activity retention during purification.
- Purity and Contamination
High purity levels of viral vectors play a critical role in maintaining both safety and functional performance. During purification, it is necessary to eliminate contaminants including culture debris, plasmid DNA, and cell-derived proteins. The similarity between packaged nucleic acid impurities and AAV vectors makes their removal difficult and these impurities present safety risks.
- Challenges in Delivery Methods
The delivery method used has a major effect on the safety and effectiveness of gene therapy treatments. The requirement for systemic delivery in diseases needing wide tissue reach results in significant immunological risks. The immune system can recognize viral vectors which leads to reduced therapeutic effectiveness and safety concerns. Active exploration continues for immunogenicity reduction techniques including capsid engineering and immune modulation.
Comparative Analysis
- Efficiency of Gene Delivery
Advanced AAV vectors along with hybrid vectors provide increased transduction efficiency together with precise tissue targeting. Engineered AAV capsids improve gene delivery to target tissues while minimizing off-target effects. Traditional AAV and lentivirus vectors demonstrate high efficiency in gene delivery yet encounter difficulties with scalability and immunogenicity.
- Safety Profiles in Preclinical Models
Preclinical studies demonstrate enhanced safety profiles for innovative vectors that feature self-inactivating elements and reduced immunogenicity. Traditional vectors provoke stronger immune reactions and present elevated risks of insertional mutagenesis especially when using lentivirus vectors.
- Suitability for Different Types of Gene Therapy Applications
Innovative vectors undergo customization to fit particular treatment applications. Cancer gene therapy utilizes hybrid vectors that integrate features from both AAV and lentivirus to achieve high efficiency alongside prolonged gene expression. Advanced AAV vectors which achieve better tissue targeting capabilities show potential for treating neurological diseases.
References
- Wang, C.; Pan, C.; Yong, H. Emerging non-viral vectors for gene delivery. J Nanobiotechnol. 2023, 21, 272. https://doi.org/10.1186/s12951-023-02044-5.
- Li, T.; Yang, Y.; Qi, H. CRISPR/Cas9 therapeutics: progress and prospects. Sig Transduct Target Ther. 2023, 8, 36. https://doi.org/10.1038/s41392-023-01309-7.
- Hardee, C.L.; Arévalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in Non-Viral DNA Vectors for Gene Therapy. Genes. 2017, 8, 65. https://doi.org/10.3390/genes8020065.
- Shi, Y.; Shi, M.; Wang, Y. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Sig Transduct Target Ther. 2024, 9, 322. https://doi.org/10.1038/s41392-024-02002-z.
- Martínez-Molina, E.; Chocarro-Wrona, C.; Martínez-Moreno, D.; Marchal, J.A.; Boulaiz, H. Large-Scale Production of Lentiviral Vectors: Current Perspectives and Challenges. Pharmaceutics. 2020, 12, 1051. https://doi.org/10.3390/pharmaceutics12111051.
- Distributed under Open Access license CC BY 4.0, without modification.
