Lentiviral Vector Development Service for Basic Research
Lentiviral vectors are engineered from the backbone of human immunodeficiency virus type-1 (HIV-1) such that accessory viral genes are systematically deleted plus long terminal repeats made self-inactivating (SIN), and since then they have become major molecular vehicles in both basic and translational biomedical research. They can carry transcriptional units over 8 kilobases, maintain high levels of transgene expression in both resting and growing cells, or be pseudotyped with envelope glycoproteins from other origins adding great practical flexibility when compared to γ-retroviral and adeno-associated viral delivery systems. In the past ten years, step-by-step changes in vector design and packaging tech—like third-gen trans-complementation systems, codon-optimized gag-pol cassettes, and microRNA-based detargeting tricks—have greatly lowered the biosafety risks tied to making replication-competent lentivirus (RCL). These advancements, in conjunction with the capacity to mediate stable genomic modifications via CRISPR/Cas9 or RNA interference modalities, have collectively established lentiviral vectors as the predominant delivery modality for functional interrogation of genes within recalcitrant cellular populations, including neuronal lineages, hematopoietic stem cells, and patient-derived organoid cultures.
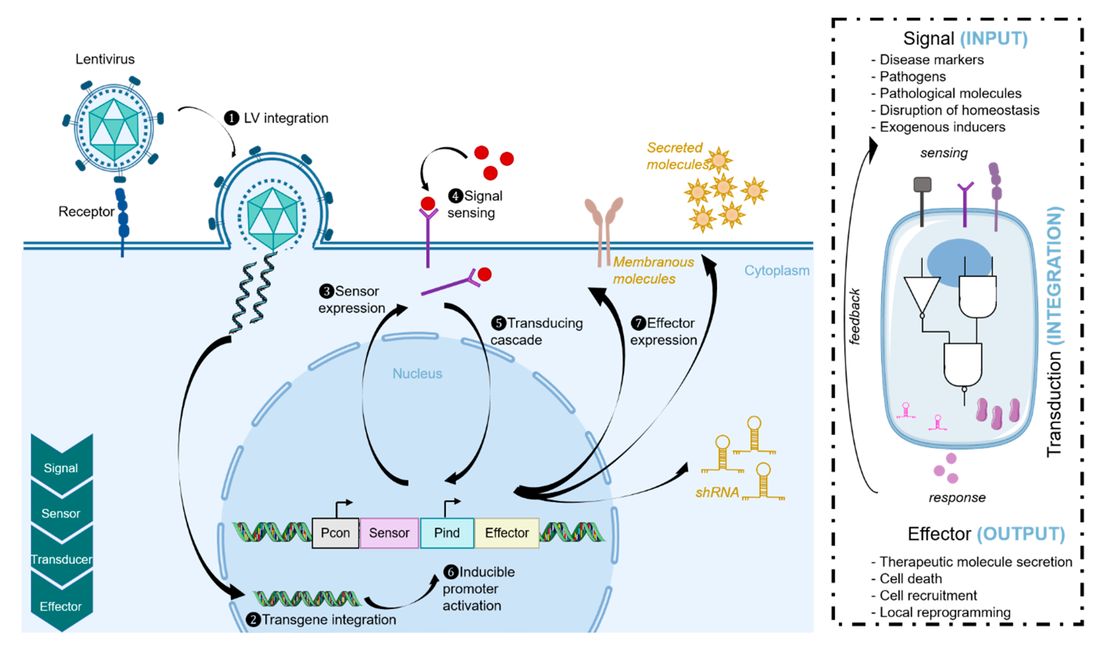 Fig. 1 Synthetic biology approaches. Lentiviral vectors are used to transduce cells1,2
Fig. 1 Synthetic biology approaches. Lentiviral vectors are used to transduce cells1,2
We recognize that translating complex genetic hypotheses into reproducible, high-titer lentiviral reagents demands a partner whose command of virology, molecular engineering, and regulatory science is both deep and seamlessly integrated. Creative Biolabs's Advanced Lentiviral Vector Development Service is purpose-built to be that single-source collaborator, dissolving every technical barrier between your initial sequence and publication-grade data. Guided by vectorology principles refined over two decades and empowered by suspension-adapted packaging lines, high-resolution titering algorithms, and orthogonal functional readouts, we convert your conceptual blueprint into precisely pseudotyped, sequence-verified lentiviral particles—ready to accelerate your discovery trajectory from benchtop design to in vivo validation without friction or delay.
Our End-to-End Service Workflow
Creative Biolabs's high-quality lentiviral vector suite integrates third-generation, suspension-adapted packaging cells with high-density transfection and proprietary ultracentrifugation-coupled chromatography to manufacture sequence-verified, replication-incompetent lentiviral particles—ready for immediate deployment in vitro or in vivo.
| Phase | Deliverables |
|---|---|
| Discovery Call & Vector Blueprint | Vector map, safety & regulatory notes, quote |
| Construct Design & Build | Sequence-verified plasmid (100 % Sanger) |
| Lentivirus Production & Titer | High-titer supernatant or concentrated stock |
| Functional QC & Data Package | MOI curve, transduction efficiency, qPCR integration profile |
| Scale-Up & Archive | Master cell bank, frozen viral aliquots, full batch record |
Our Core Service Modules
Vector Design & Strategy – What We Custom-Build for You
Your therapeutic intent defines the destination; we architect the lentiviral vector that navigates the genomic landscape to reach it. Beginning with mechanistic modeling of your transgene's expression cassette and continuing through scalable, high-quality production, every design parameter—promoter strength, envelope tropism, insulator placement, and safety switch configuration—is optimized against the molecular signature of your target cell and the clinical timeline of your indication. Regulatory strategy is not an afterthought; it is embedded as a design criterion from day zero, ensuring that each decision—vector backbone, packaging system, and release assay—anticipates CMC expectations and accelerates your path to the clinic.
- Promoter Selection
You choose the expression profile; we engineer the switch. Constitutive powerhouses – CMV for maximal output in HEK293, EF1α for immune cells, CAG for broad tissue coverage. Fine-tuned tissue specificity-Syn1 (neurons), GFAP (astrocytes), CD68 (microglia/macrophages), Albumin (hepatocytes), or any promoter from our 200-member library. Inducible cassettes – we optimize the TRE spacing and insulator sequences to eliminate basal leak. In the short term, you receive a promoter activity heat-map in your cell line, plus a ranked recommendation list.
- Multi-cistronic Architectures
Need one vector to do three jobs? We do the stoichiometry math for you. 2A peptide linkers (P2A, T2A, E2A) – balanced cleavage efficiency validated by LC-MS. IRES elements – strong (EMCV) for equimolar dual expression or attenuated (CrPV) when downstream gene must lag. "All-in-one" CRISPR arrays – Cas9-2A-BFP-U6-sgRNA-WPRE in 7.8 kb; we remove cryptic poly-A sites and predict gRNA secondary structure to preserve cutting efficiency. Annotated SnapGene file plus predicted cleavage ratios and experimental validation data from a pilot transduction.
- Safety Modifications
Regulatory reviewers love clean genomes. We handle the details so you don't rewrite your IBC protocol. Third-generation SIN LTRs – deletion of U3 enhancer blocks RCL formation; we sequence-verify both 5′ and 3′ junctions. WPRE excision on request – reduces insert size and eliminates residual enhancer risk for in vivo dosing. Self-inactivating 3′ LTRs – further attenuates promoter interference; optional addition of chromatin insulator cHS4. Full biosafety dossier (sequence alignments, RCL assay plan) ready for institutional review.
- Fluorescent & Selection Markers
Pick your color, pick your drug, or pick both. Fluorophores – mScarlet, BFP2, far-red iRFP720; codon-optimized for human expression and tested for spectral bleed-through. Antibiotic resistance – PuroR, BlastR, NeoR, Hph (hygromycin) with attenuated promoters to avoid over-stressing primary cells. Surface tags – HA or Myc for live-cell sorting with magnetic beads; includes epitope accessibility modeling. Side-by-side fluorescence intensity chart and kill-curve data in your target cell type within a short time.
Cloning & Sequence Verification – How We Turn Your Idea into a Ready-to-Use Plasmid
- Flexible Input – Start Where You Are
Have nothing but a FASTA? We'll design promoters, Kozak context, and poly-A signal. Hand-drawn sketch on a napkin? Our bioinformatics team will convert it into an annotated GenBank file in the short term. Existing plasmid that keeps failing? Send the samples; we'll sub-clone the cassette into a high-yield lentiviral backbone while preserving restriction sites you love. Editable SnapGene file + design rationale report—before we touch a pipette.
- Golden-Gate & Gibson Assembly – Seamless, Scar-Free
Golden-Gate for multi-fragment builds (promoter-ORF-marker in one pot). We optimize overhangs with thermodynamic modeling to give >95 % correct colonies. Gibson Assembly for complex inserts >7 kb; we run a proprietary 3-step annealing program that halves the typical error rate. Zero scar junctions mean no unwanted amino acids in fusion proteins—critical for crystallography or antibody labeling. Colony PCR screen data and mini-prep QC gel the same day assembly is complete.
- 100 % Sanger Verification – Nothing Ships Without Proof
Entire insert, both ITRs, and every junction are covered by overlapping primers. We cross-check against your original design with automated variant calling; you receive a color-coded alignment. If any SNV or indel appears, we re-sequence the batch at no cost—and re-build if the error frequency exceeds 0.05 %. Signed sequence certificate ready for patent filing or journal submission.
- Optional Codon-Optimization – Maximize Expression, Minimize Risk
Species-specific algorithms for human, mouse, or rat; we balance CAI, mRNA secondary structure, and rare tRNA availability. Cryptic splice-site removal predicted by MaxEntScan; eliminates aberrant transcripts before they start. Immunogenic epitope depletion for in vivo applications—validated by NetMHCpan. Before-and-after expression bar charts in your target cell line, plus a one-page rationale for reviewers.
Lentivirus Production & Concentration – What You Get & Why It Matters
- Pseudotyping Menu – Pick the Envelope, We Ship the Trope
VSV-G (standard): broadest host range; ideal for screening panels or when cell tropism is unknown.
Rabies-G: ≥10-fold higher neuron transduction than VSV-G; validated in cortical, hippocampal, and dorsal-root-ganglion cultures.
BaEV-R (baboon endogenous virus): unlocks human CD34⁺ HSPCs and primary T-cells without cytokine pre-activation.
RD114: feline endogenous envelope engineered for human T-cell and NK-cell targeting; perfect for CAR-T workflows.
Need something niche? Send us the envelope sequence—our glycoprotein swap pipeline delivers a custom pseudotype in the short term. Side-by-side transduction heat-map in your cell line plus MOI recommendations.
| Scale | Typical Titer | Volume | Application |
|---|---|---|---|
| Mini (research) | 1×107 TU/mL | 1 mL | Pilot transduction |
| Midi | 1×108 TU/mL | 5 mL | In vitro screens |
| Maxi (pre-clinical) | 1×109 TU/mL | 10–50 mL | In vivo mouse/rat studies |
- Endotoxin Removal – Clinic-Ready Purity
Dual-step process: Triton X-114 phase separation strips >90 % endotoxin, followed by anion-exchange polishing to <0.1 EU/µg.
Lot-release certificate includes raw LAL chromogenic data—no re-test required at your vivarium.
Optional endotoxin "ultra-low" grade (<0.05 EU/µg) for sensitive in vivo studies; quoted upfront.
Endotoxin report attached to every shipment; if it fails spec, we remake the batch free of charge.
- Functional Titering – Know the Exact Dose
qRT-PCR (TU/mL): measures integrated provirus copies shortly after transduction; linear range 104–109 TU/mL.
Flow-cytometry (GFP or mCherry): real-time readout of % positive cells at defined MOIs; includes viability gating.
Dual-method validation for every lot—discrepancies >20 % trigger automatic re-titration. Titer certificate, raw FCS files, and a ready-to-use MOI calculator spreadsheet so you can plate your experiment the same afternoon your virus arrives.
Functional Validation Suite
- Transduction Efficiency – We Optimize Your MOI Before You Spend a Cell
10-point MOI matrix (0.1–10) in your exact cell line, quantified by high-resolution flow cytometry (GFP/mCherry) with live/dead gating. An interactive MOI-response curve + recommended working MOI to hit 70–90 % positive cells with <5 % viability loss. Need rare or primary cells? We'll run the same assay on your patient-derived organoids or CAR-T blasts—no extra hands required.
- Integration Profile – Know Your Copy Number, Not Your Guess
Droplet digital PCR (ddPCR) against the lentiviral WPRE element and a reference single-copy gene; sensitivity down to 0.1 copies per diploid genome. Copy-number histogram and statistical summary; ideal for clonal selection or appropriate biodistribution packages. If your protocol demands monoclonal lines, we'll sort and re-quantitate integration for each clone—results in time.
- Off-Target Scanning – CRISPResso2 Report Ready for Reviewers
Amplicon-seq of up to 20 predicted off-target loci; CRISPResso2 pipeline delivers indel frequencies, frameshift ratios, and visual allele plots. Annotated report with QC metrics and a concise "off-target risk statement" for your manuscript. Discrepancy with in-silico predictions? We'll re-design the sgRNA and re-screen at no charge in the short term.
- Toxicity Panel – Prove Your Vector Is Safe Before Animal Work
The detection was carried out using the activity assay; IC50 calculated against mock-transduced controls. IFN-β ELISA in human THP-1 cells to rule out innate immune activation (<10 pg/mL cutoff). Annexin V apoptosis staining, cytokine 10-plex, or oxidative-stress ROS assay. Signed safety certificate + raw luminescence/ELISA files formatted for your IACUC or IRB submission.
Why Choose Us?
Vector Design Intelligence
PhD-level vector engineers integrate directly with your discovery team, converting therapeutic hypotheses into a rationally engineered lentiviral genome. We model promoter strength, envelope tropism, and safety-switch placement in silico, then benchmark designs against off-target and immunogenicity profiles. Iterative data packages arrive every week, giving leadership quantitative go / no-go criteria without expanding internal headcount.
End-to-End Solutions
Whether you arrive with a raw FASTA or a locked date, we scale from research-grade supernatant to drug substance without hand-offs. High-titer vectors are released shortly after sequence locking; high-quality batches follow a harmonized protocol that consolidates upstream production, downstream purification, and biodistribution/toxicology under one program-managed Gantt chart synchronized to your eCTD milestones.
Regulatory-Ready Quality Gates
Each high-quality vector lot is certified against a 30-attribute quality panel that quantifies functional titre (qPCR and flow cytometry), particulate load (nanoparticle tracking analysis), residual plasmid/host DNA (droplet digital PCR), endotoxin (< 1 EU/mL), and the absence of replication-competent lentivirus (qPCR assay, ≥ 106-fold analytical sensitivity). Raw analytical outputs are collated within pre-structured regulatory dossier segments—certificate of analysis.
Modular Architecture, Bespoke Payload
No off-the-shelf backbones. Each construct is configured de novo: self-inactivating LTRs, envelope libraries (VSV-G, RD114-TR, BaEV-R, measles, or mosaic variants), and tissue-restricted or inducible promoters selected via barcoded screening in primary human cells. Plug-and-play cassettes allow rapid swaps of transgenes, promoters, or safety switches.
Client Testimonials

Frequently Asked Questions
Q: Why Lentiviral Vectors in Basic Research?
A: Stable gene integration for long-term expression in dividing and non-dividing cells
Unlike γ-retroviral vectors that require host-cell division for nuclear entry, lentiviral vectors exploit the HIV-1 pre-integration complex, enabling active nuclear import and subsequent chromosomal integration. Once integrated, the transgene is inherited by all progeny, ensuring durable expression that can be monitored for weeks to months without re-administration. This permanence is critical for lineage-tracing experiments and chronic disease modeling where transient expression would obscure phenotypes.
Large cargo capacity (up to 10 kb) for multi-gene circuits, CRISPR libraries, and reporters
The lentiviral genome tolerates inserts of 7–10 kb—substantially more than AAV (≈4.5 kb)—permitting polycistronic cassettes such as CRISPR-Cas9-2A-fluorophore-sgRNA arrays or multi-reporter systems (e.g., dual-luciferase plus antibiotic-resistance markers). Consequently, pooled CRISPR libraries exceeding 100 000 sgRNAs can be delivered at low MOI, achieving single-integrant cells that simplify downstream NGS deconvolution.
Broad tropism
Substitution of the native VSV-G envelope with measles-H/F, BaEV-R, or RD114 glycoproteins redirects tropism to different models without altering the viral genome. Such "plug-and-play" pseudotyping has been instrumental in generating CAR-T cells, transducing human cerebral organoids, and targeting microglia within intact brain explants.
Q: Which transfection chemistry should I specify for scalable, high-titer lentivirus production?
A: Polyethylenimine (PEI)-mediated transfection has become the benchmark for scalable lentiviral vector (LV) manufacturing. In head-to-head comparisons, PEI reproducibly yields functional titers (≥1 × 107 TU/mL) that are statistically equivalent to calcium-phosphate precipitation, while requiring 10-fold less plasmid DNA and exhibiting minimal batch-to-batch variability. The method is pH-insensitive and can be executed in serum-free medium, facilitating direct downstream processing.
Q: What is the optimal DNA:PEI ratio for maximal lentivirus vector yield?
A: Design-of-experiments (DoE) studies across 24-parallel micro-bioreactors identified a DNA:PEI mass ratio of 1:3 as the critical parameter, yielding ≥1 × 108 TU/mL in 2 L stirred-tank bioreactors. Deviations >20 % from this ratio significantly decreased infectious titers due to increased cytotoxicity.
Q: Can the process be directly scaled from 24-well plates to 50 L bioreactors?
A: Yes. Using DoE-guided optimization in automated ambr 15 systems, critical process parameters (cell density, DNA:PEI ratio, harvest window) were locked and successfully translated to a 50 L BioSTAT STR, achieving identical infectious titers (1.3 ± 0.1 × 107 TU/mL) and <10 % batch-to-batch variability.
References
- Page, Audrey, Floriane Fusil, and François-Loïc Cosset. "Toward tightly tuned gene expression following lentiviral vector transduction." Viruses 12.12 (2020): 1427. https://doi.org/10.3390/v12121427.
- Distributed under Open Access license CC BY 4.0, without modification.
