All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Characteristics of Human PBMC
Peripheral blood mononuclear cells (PBMC) are a subpopulation of leukocytes, which serve as the dominant cells of the body's immunity system that generate selective responses to the immune system against diseases. The position of PBMCs is very important as they serve as a defense line against infection and disease. PBMC cells are usually isolated from whole blood samples. PBMCs consist of a variety of specialized cell populations, such as monocytes, lymphocytes, macrophages, and dendritic cells, these different cells play specific functions and are used in medical fields.
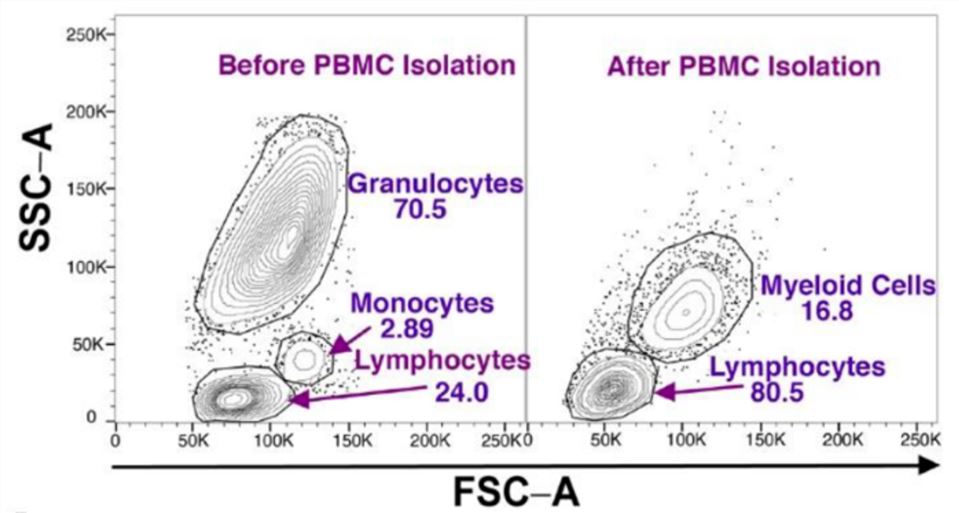 Fig.1 PBMC subsets identified by flow cytometry analysis.1
Fig.1 PBMC subsets identified by flow cytometry analysis.1
PBMC Isolation
Generally, there are two main PBMC isolation methods, ficoll-density gradient centrifugation and red blood cell lysis. Ficoll-density gradient centrifugation is a cheap and simple method for PBMC isolation. Ficoll is a type of hydrophilic polysaccharide used to separate the blood layers. Ficoll-density gradient centrifugation separates the blood into the top layer of plasma, followed by the bottom layer of PBMCs, then a subset of polymorphonuclear cells (such as eosinophils and neutrophils), and finally the bottom red blood cells layer.
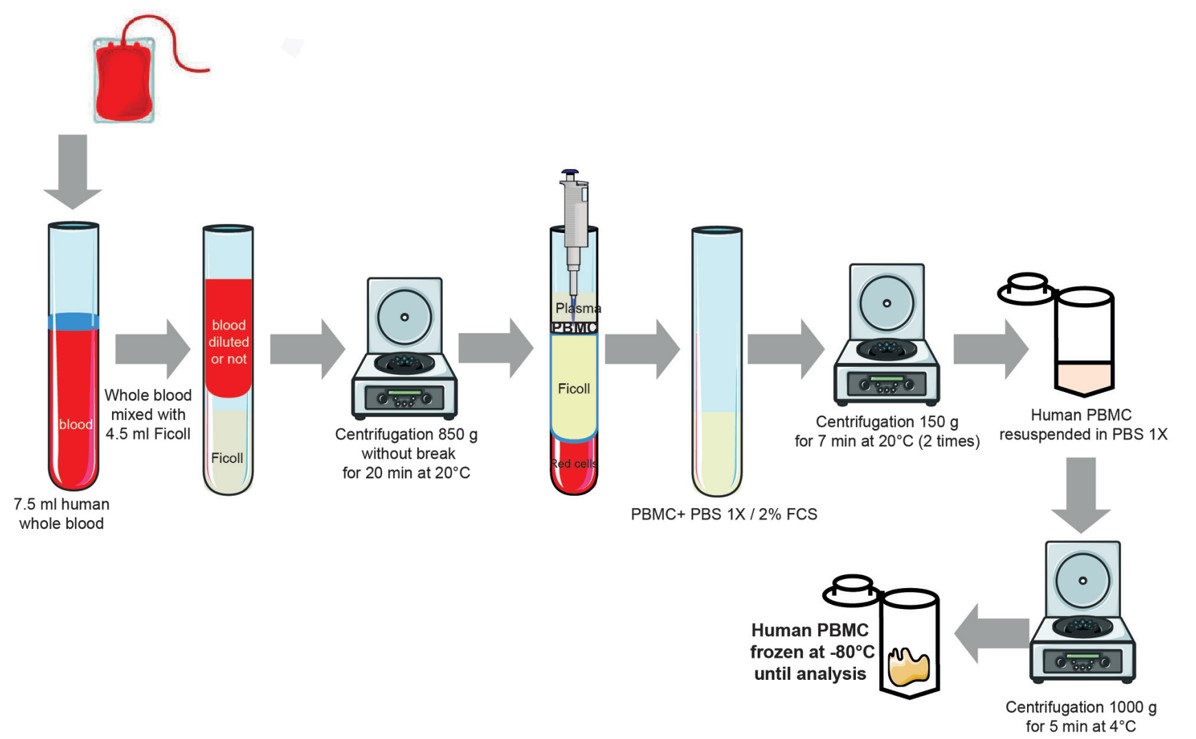 Fig.2 Experimental protocol for isolating human peripheral blood mononuclear cells from whole blood.2
Fig.2 Experimental protocol for isolating human peripheral blood mononuclear cells from whole blood.2
The Applications of PBMCs
Compared with isolated cells, PBMCs as an integral cell population that plays a critical role in the constructing and maintaining effective immune response. Based on the feature, several researchers use PBMC to exploit the immune responses of drug candidates. There are multiple research areas applications for PBMCs including:
PBMCs Cells at Creative Biolabs
In order to better help global customers achieveing the success of the project, Creative Biolabs provides a variety of isolated Peripheral Blood Mononuclear Cells (PBMCs) from whole blood obtained from IRB-consented donors. Our ready-for-use PBMC products are offered with strict and standardized QC data. Meanwhile, we are committed to providing customized cell products according to global customers' variable needs. Our PBMC cell products with the following features:
Here are some hot cell products you can browse to find a suitable product for your project. For more defined requirements, please feel free to contact us or send us an inquiry.
References
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION