Chimeric antigen receptor T cell (CAR-T) therapy has emerged as a major treatment for aggressive B cell tumors, with potential for additional malignancies. However, its high production costs, which are caused by single-use reagents for T cell activation and clinical-grade viral vectors, prevent its widespread application. The development of a cell-based artificial T cell stimulator that allows for continuous T cell stimulation might provide a cost-effective and efficient alternative to CAR-T cell activation, thereby reducing production costs and broadening access to this therapy.
Improved T cell activation
Artificial T cell stimulators can more closely mimic the natural interactions between T cells and antigen-presenting cells (like dendritic cells), leading to a more physiological and potentially more effective T cell activation.
Reduced manufacturing costs
Artificial T cell stimulators can more closely mimic the natural interactions between T cells and antigen-presenting cells (like dendritic cells), leading to a more physiological and potentially more effective T cell activation.
Enhanced T cell function
Studies suggest that artificial T cell stimulators-activated CAR T cells may exhibit improved characteristics, such as increased potency and persistence in the body.
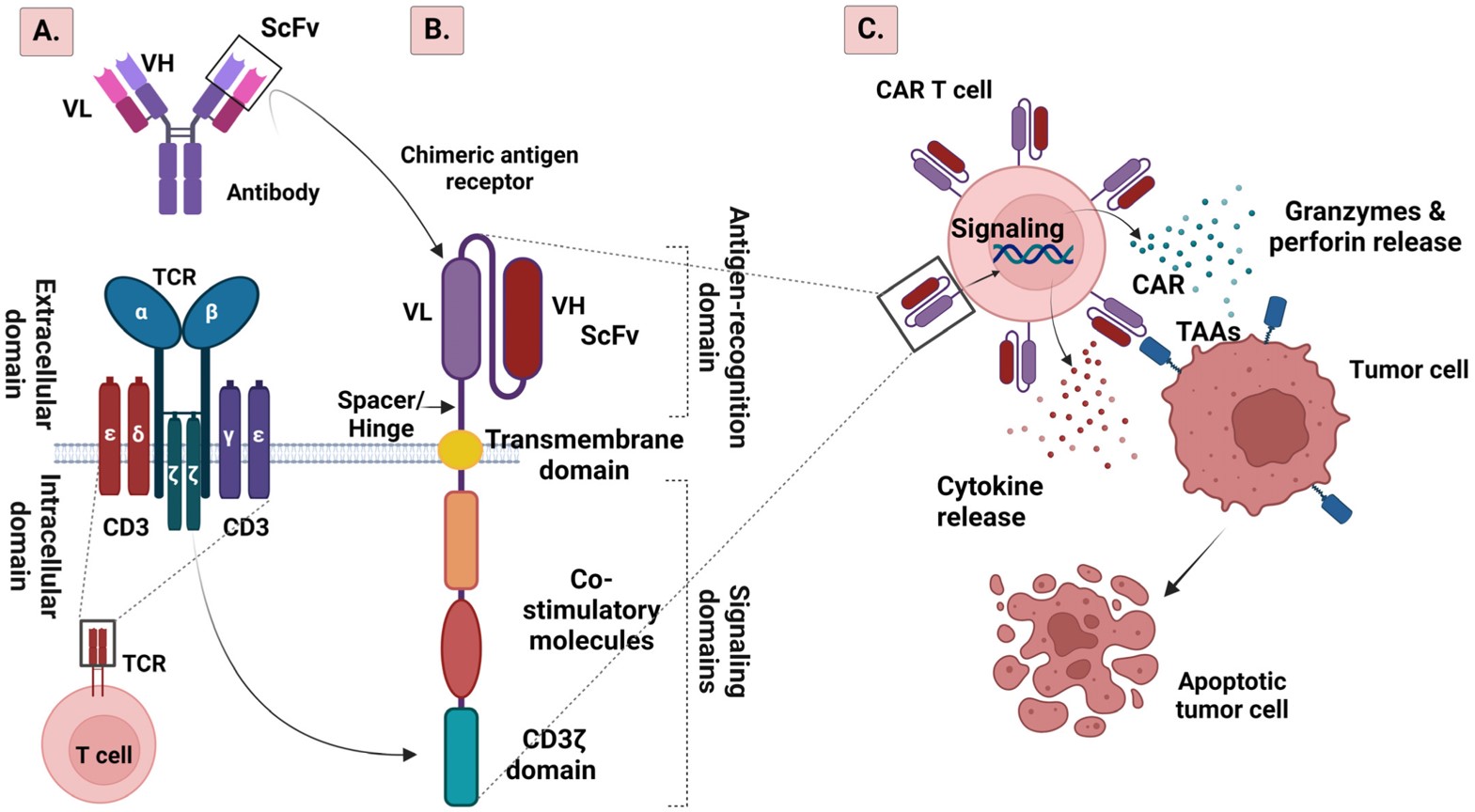 Fig.1 The structure of TCR and CAR as well as CAR-T cell therapy mechanism.1,3
Fig.1 The structure of TCR and CAR as well as CAR-T cell therapy mechanism.1,3
Creative Biolabs globally delivers a cost-effective chimeric antigen receptor transduced T cell based artificial T cell stimulator development service, aiming to improve CAR-T cell immunotherapy and broaden its application. Our offerings encompass the totality of the process, ranging from the construction of the artificial T cell stimulator, and the optimization of the artificial T cell stimulator to the functional characterization of artificial T cell stimulator and activated CAR-T cells. With cutting-edge cell engineering technologies and stringent quality assurance protocols in place, we are capable of swiftly and efficiently producing high-performance artificial T cell stimulators, enabling accurate activation and expansion of CAR-T cells.
Furthermore, we offer customized experimental design and data analysis services to meet the specific needs of each customer. We are dedicated to working hand in hand with our customers to advance CAR-T cell immunotherapy into clinical practice.
Featured Advantages
Background: Autologous CD19 CAR T-cell treatments have been extremely effective in treating specific blood malignancies. However, their efficacy can be hampered by limited and uneven T-cell functioning, which varies greatly between patients. This heterogeneity is determined by factors such as the patient's underlying health status and the quality of the initial T-cell population.
Method: This study investigated the impact of a novel artificial T cell stimulator scaffold on CAR T-cell functionality. This innovative artificial T cell stimulator platform utilizes a physiological fluid bilayer to precisely deliver critical T-cell stimulatory ligands, namely anti-CD3 and anti-CD28, at well-defined densities. A diverse set of blood samples was collected, including those from healthy donors and patients with acute lymphoblastic leukemia (ALL) or chronic lymphocytic lymphoma (CLL), representing a spectrum of patient health status. These samples were then used to generate a library of CAR T-cell products using the artificial T cell stimulator scaffold.
Results: By employing this synthetic artificial T cell stimulator platform, the study successfully generated a library of CAR T-cell products from both healthy and patient blood samples (ALL and CLL). This platform, with its ability to precisely control the delivery of T-cell stimulatory signals, provides a valuable tool for investigating and potentially optimizing CAR T-cell production and function across diverse patient populations.
 Fig.2 The quality and potency of generated CAR-T cells are directly determined by the strength and nature of the signals received during their activation.2,3
Fig.2 The quality and potency of generated CAR-T cells are directly determined by the strength and nature of the signals received during their activation.2,3
Creative Biolabs offers a wide range of specialized artificial T cell stimulator development services, not just for CAR-T cells but also for other immune cell types. Our services include the customization of artificial T cell stimulators for CD4+ T cells, CD8+ T cells, CD3+ T cells, NK cells, and others. This comprehensive range expands our capabilities to assist a wide range of immunological research and therapeutic applications, ensuring that we satisfy the needs of our customers' individual demands in progressing their projects.
For further information, please follow the link to contact us about creating a customized, flexible artificial T cell stimulator for CAR-transduced T cell stimulation.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION