Artificial T cell stimulators represent a significant advancement in NK cell immunotherapy by providing a controlled and robust platform for NK cell expansion and activation. These stimulators effectively mimic the interactions between T cells and antigen-presenting cells, driving robust NK cell proliferation while maintaining their cytotoxic function, which enables the generation of large numbers of potent NK cells from limited sources and allows for precise control over the activation and differentiation of these cells. The versatility of artificial T cell stimulators, combined with their potential for clinical translation, makes them a promising strategy for developing more effective and personalized NK cell-based therapies against a range of malignancies.
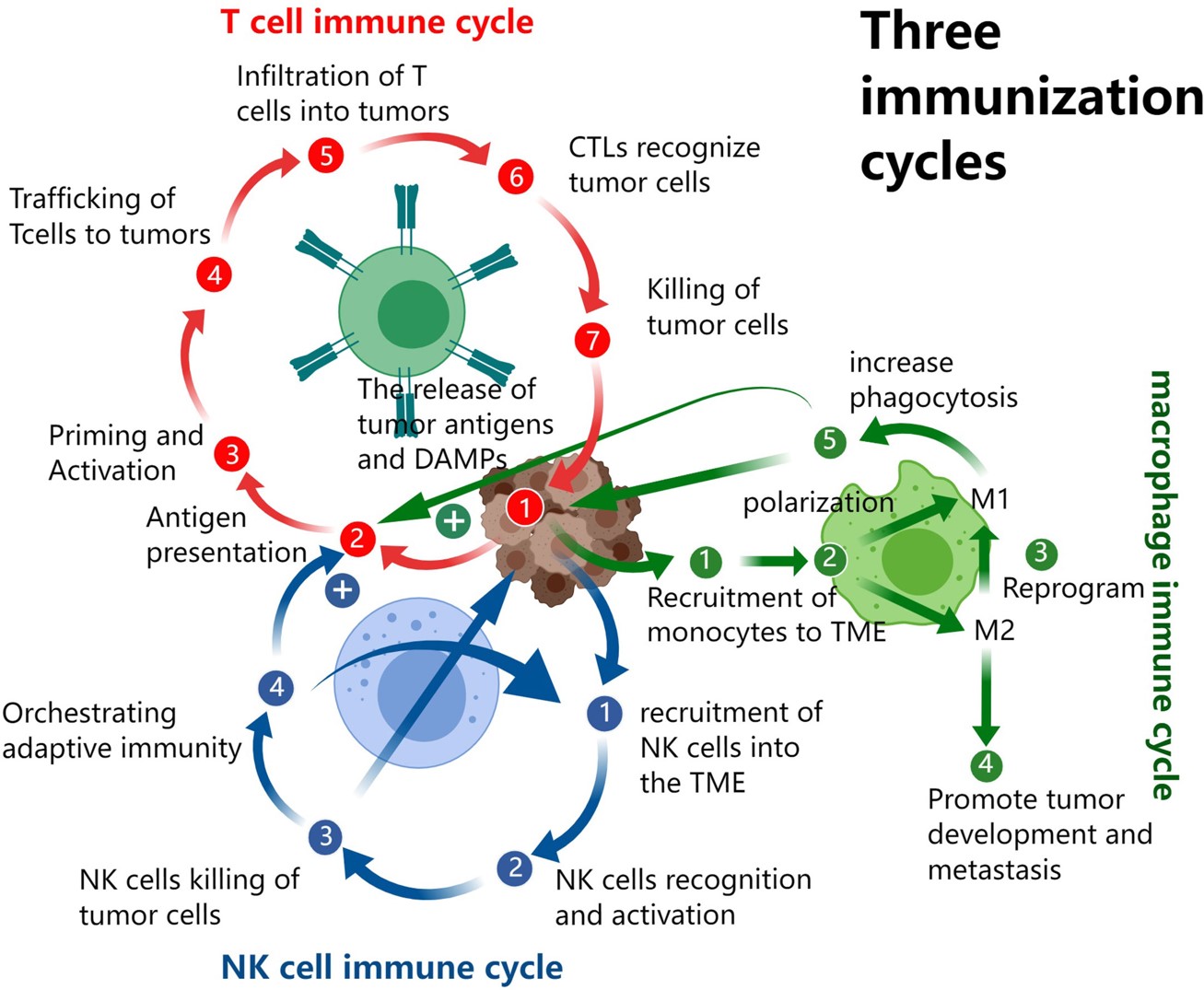 Fig.1 Cancer immunity cycles depend on T cells, NK cells, and TAMs.1,3
Fig.1 Cancer immunity cycles depend on T cells, NK cells, and TAMs.1,3
Creative Biolabs provides a specialized NK cell-based artificial T cell stimulator development service to empower NK cell-based immunotherapies. Our expertise in immunology and cell biology enables us to design and engineer artificial T cell stimulators to precisely mimic natural antigen-presenting cells, achieving homogeneous NK cell activation through tailored delivery of specific activation signals. At the same time, we provide end-to-end support, from initial design and engineering to rigorous functional validation, ensuring efficient and high-quality outcomes. Our flexible approach allows for customized stimulator designs and precise control over stimulation parameters, accelerating scientific progress and facilitating the rapid development of novel NK cell-based immunotherapies for our clients.

Background: NK cells are crucial for anti-tumor immunity, including against multiple myeloma (MM). Umbilical cord blood (CB) is a valuable source of NK cells for allogeneic therapies. However, the effective clinical application requires a significant expansion of CB-derived NK (CB-NK) cells.
Method: This study developed a novel method for expanding CB-NK cells using artificial stimulators in a gas-permeable culture system. CB units, both fresh and cryopreserved, were utilized. NK cell expansion was assessed over 14 days, and the purity, phenotype, and cytotoxicity of the expanded CB-NK cells were thoroughly characterized.
Results: The artificial stimulator-based method achieved substantial NK cell expansion, with an average fold increase of 1848 from fresh and 2389 from cryopreserved CB. Expanded CB-NK cells exhibited high purity (>95% CD56+/CD3−) and maintained a functional phenotype with preserved cytotoxicity against various MM targets. Importantly, artificial stimulator-expanded CB-NK cells demonstrated significant in vivo anti-MM activity in a xenograft mouse model.
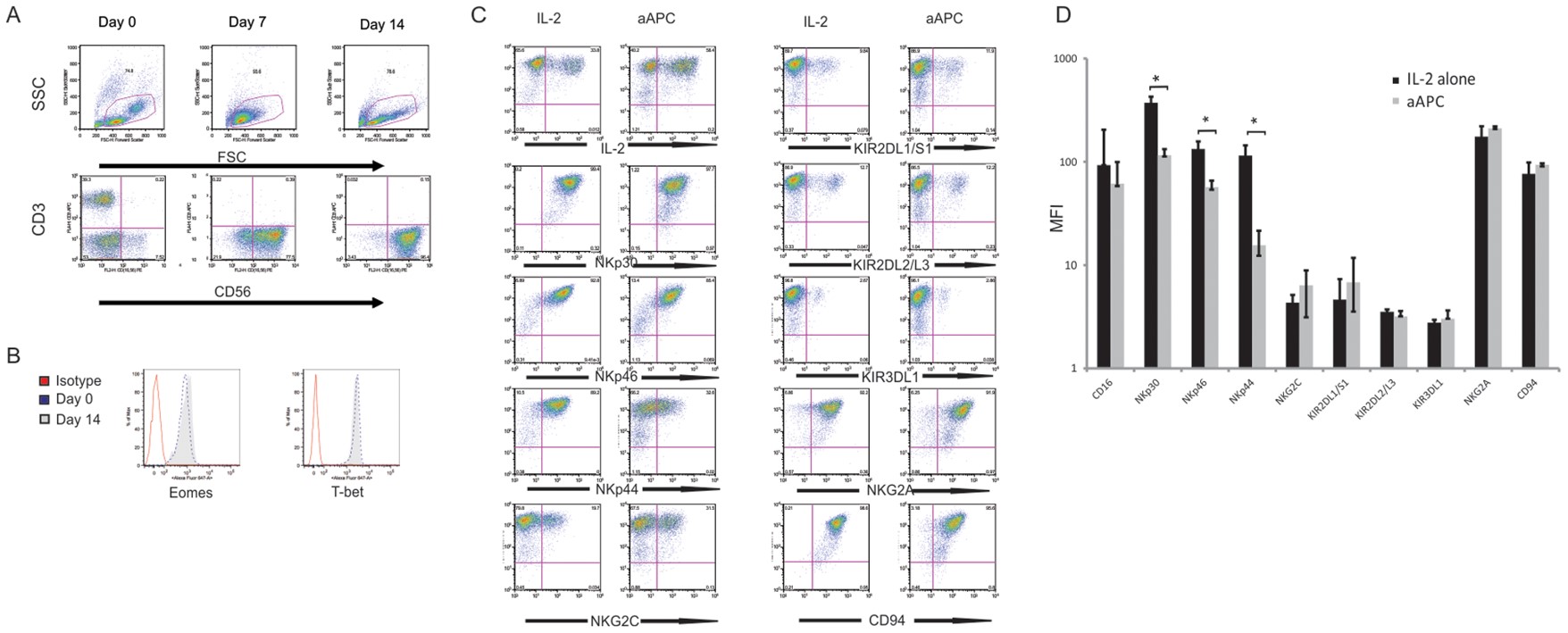 Fig.2 CB-NK cell phenotype in response to artificial stimulants.2,4
Fig.2 CB-NK cell phenotype in response to artificial stimulants.2,4
 Fig.3 CB-NK cells generated by artificial stimulators create immunological synapses with myeloma targets and are cytotoxic to them.2,4
Fig.3 CB-NK cells generated by artificial stimulators create immunological synapses with myeloma targets and are cytotoxic to them.2,4
To support a variety of immunological research applications, Creative Biolabs also provides a comprehensive range of specialized artificial T cell stimulator development services for other subtypes, which include:
Please get in touch with us and discuss your individual project needs if you would like to discuss the creation of a flexible, bespoke artificial T cell stimulator designed for NK cell stimulation.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION