Chimeric antigen receptor (CAR) T cell therapy has transformed the landscape of cancer treatment, particularly for B-cell malignancies. Traditional methods employing viral vectors for transgene delivery are impeded by high costs and stringent regulatory constraints, limiting their broader clinical application. At Creative Biolabs, we are pioneering a novel approach with our CellRapeutics™ Non-viral In Vivo CAR Technology, which aims to bridge these gaps by leveraging non-viral vectors for in vivo CAR-T cell generation.
Non-viral Vectors: An Alternative Paradigm
Non-viral vectors have gained significant attention, especially with the widespread deployment of mRNA-based vaccines for SARS-CoV-2. At Creative Biolabs, our CellRapeutics™ platform utilizes lipid nanoparticles (LNPs) and polymer-based nanoparticles to deliver CAR transgenes directly into T cells in vivo. These particles, unlike their viral counterparts, rely on their physicochemical properties for cell entry, trafficking, and transgene delivery. This method not only reduces the complexity and cost associated with ex vivo cell engineering but also holds the potential for broader clinical applications.
Nanoparticle Design Strategies
Our technology employs LNPs composed of ionizable cationic lipids, helper lipids (phospholipids and cholesterol), and polyethylene glycol (PEG)-conjugated lipids. These LNPs are designed to shield the nucleic acid cargo from degradation and facilitate its delivery to target cells via endocytosis. To enhance specificity, the LNPs can be decorated with targeting antibodies using SATA-maleimide chemistry. Similarly, our biodegradable PBAE polymer formulations exploit electrostatic interactions to assemble with anionic nucleic acids and undergo pH-dependent endosomal escape, ensuring efficient delivery to T cells.
We provide a variety of product types such as IVT products (IVT-mRNA, IVT-mRNA-LNP) to facilitate in vivo CAR engineering, meanwhile, multi-step optimization is carried out during the production process. During the instantaneous CAR expression process of IVT mRNA, mRNA is optimized with elements such as 5' caps, 5' and 3' untranslated regions (UTRs), and poly(a) tail to improve stability and translation efficiency.
We provide comprehensive services for the in vivo production of CAR-T cells, from nanoparticle synthesis and antibody conjugation to in vivo testing and validation. Our state-of-the-art facilities ensure high-quality and reproducible results.
Our preclinical and clinical support services encompass pharmacokinetic and pharmacodynamic studies, safety evaluations, and regulatory consulting. We assist clients in navigating the complex regulatory landscape to expedite the translation of their therapies from bench to bedside.
Numerous studies support the efficacy and safety of non-viral vectors for CAR-T cell generation. For example, preclinical research has shown a promising alternative to traditional T cell engineering by using IVT mRNA-loaded nanocarriers to reprogram T cells in vivo, producing functional CAR-T cells with antitumor activity.
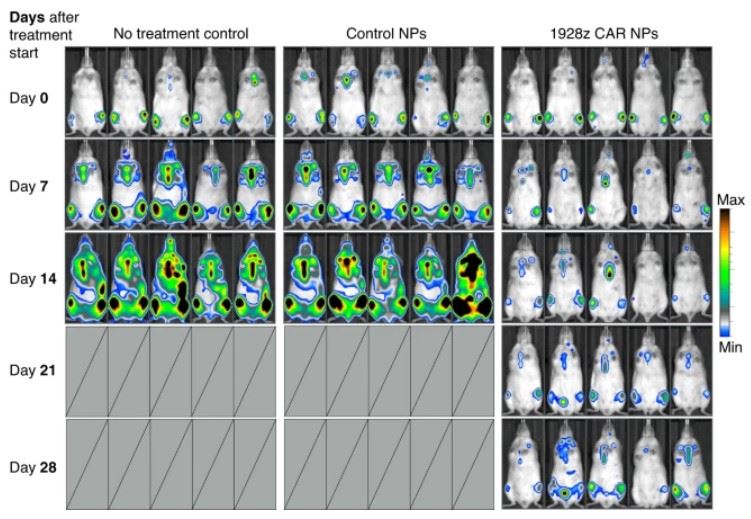 Fig.1 The anti-tumor activities of mRNA nanoparticle-redirected T cells in fully immunocompetent hosts.1
Fig.1 The anti-tumor activities of mRNA nanoparticle-redirected T cells in fully immunocompetent hosts.1
Q1: What are the main advantages of non-viral in vivo CAR technology?
A1: The main advantages include reduced costs, enhanced safety, scalability, and the ability to rapidly deploy therapies without the need for complex ex vivo cell engineering processes.
Q2: How does Creative Biolabs ensure the specificity of CAR delivery?
A2: We employ advanced targeting strategies, such as decorating nanoparticles with antibodies specific to T-cell markers, to ensure precise delivery and uptake by the desired cells.
Our CellRapeutics™ Non-viral In Vivo CAR Technology offers a safe, cost-effective, and scalable alternative to viral vector-based approaches. Through continuous research and collaboration, we are committed to bringing these life-saving therapies to patients worldwide. For more information, please contact us directly.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION